
��ע�⣺���������������Ч��
������A��C11H8O4��������������Һ�м��ȷ�Ӧ�����ữ�ɵõ�������B��C���ش��������⣺
��1�� B�ķ���ʽΪC2H4O2��������ֻ��һ�������š���B�Ľṹ��ʽ��________��B���Ҵ���Ũ������¼��ȷ�Ӧ����D���÷�Ӧ�Ļ�ѧ����ʽ��________________________���÷�Ӧ��������________��д�������ܷ���������Ӧ��B��ͬ���칹��Ľṹ��ʽ________________________��
��2�� C�Ƿ��㻯�����Է�������Ϊ180����̼����������Ϊ60.0���������������Ϊ4.4��������Ϊ������C�ķ���ʽ��________��
��3����֪C�ķ�����������ȡ����������һ��ȡ������֧�����һ�����ʹ������Ȼ�̼��Һ��ɫ�Ĺ����ż�����̼��������Һ��Ӧ�ų�����Ĺ����ţ����ȡ�����ϵĹ�����������
����������ȡ������ͬ���ֱ�λ�ڸ�ȡ��������λ�Ͷ�λ����C�Ľṹ��ʽ�� ��
(4)A�Ľṹ��ʽ�� ��
���𰸡�(1)CH3COOH CH3COOH+ CH3CH2OH![]() CH3COO CH2CH3+H2O ������Ӧ��ȡ����Ӧ�� HCOOCH2CH2CH3 HCOOCH(CH3)2
CH3COO CH2CH3+H2O ������Ӧ��ȡ����Ӧ�� HCOOCH2CH2CH3 HCOOCH(CH3)2
(2) C9H8O4
(3)̼̼˫�� �Ȼ� CH=CHCOOH 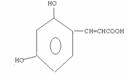
��4��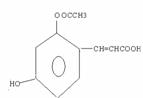
����������1��B�ķ���ʽΪC2H4O2��������ֻ��һ�������š���B�Ľṹ��ʽ��CH3COOH ��CH3COOH���Ҵ���Ũ������¼��ȷ�Ӧ�Ļ�ѧ����ʽ��CH3COOH+ CH3CH2OH![]() CH3COO CH2CH3+H2O�÷�Ӧ��������ȡ����Ӧ���ܷ���������Ӧ�ģ�˵��B��ͬ���칹���к���ȩ������ṹ��ʽΪHCOOCH2CH2CH3 HCOOCH(CH3)2 ��2��C�Ƿ��㻯���˵��C�к��б�������Է�������Ϊ180����̼����������Ϊ60.0������̼Ϊ9�������������Ϊ4.4��������Ϊ8������Ϊ����Oԭ�Ӹ���Ϊ4����C�ķ���ʽ��C9H8O4
CH3COO CH2CH3+H2O�÷�Ӧ��������ȡ����Ӧ���ܷ���������Ӧ�ģ�˵��B��ͬ���칹���к���ȩ������ṹ��ʽΪHCOOCH2CH2CH3 HCOOCH(CH3)2 ��2��C�Ƿ��㻯���˵��C�к��б�������Է�������Ϊ180����̼����������Ϊ60.0������̼Ϊ9�������������Ϊ4.4��������Ϊ8������Ϊ����Oԭ�Ӹ���Ϊ4����C�ķ���ʽ��C9H8O4
��3��C�ķ�����������ȡ����������һ��ȡ������֧�����һ�����ʹ������Ȼ�̼��Һ��ɫ�Ĺ�����˵������̼̼˫��������̼��������Һ��Ӧ�ų�����Ĺ����ţ�˵�������Ȼ�����������ȡ������ͬ�����ݷ���ʽ�������������Ĺ�����Ϊ�ǻ�����ֱ�λ�ڸ�ȡ��������λ�Ͷ�λ����C�Ľṹ��ʽ��
��4��A�Ľṹ��ʽ��
�����㡿�������漰���л������ʡ��л������š�ͬ���칹����������д���л���Ӧ����ʽ����д�����ص㿼��ѧ��˼ά�������Ժ�����ԡ�
�������������͵��л����⣬ȫ�濼�����л�����ʽ�ͽṹ��ʽ���Ƶ����ṹ��ʽ�ͻ�ѧ����ʽ����д��ͬ���칹����ж�����д��ȫ�濼����ѧ��˼ά��������������ͽ�������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ��������������Թ��У�������������ˮ�������ʹ̼�����ȫ�ܽ⣮���벻�ܹ�������Һ��������ˮ���ϴ�Ӳ��ܹ��壮 | ������ﲿ���ܽ⣮ |
| ����2�����Թ��м������� CuSO4 CuSO4 ��Һ���ټ����������ܹ��壬����� |
��1������Һ��ɫ������IJ��ܹ��������Ա仯������� 2 2 ��������2������Һ��ɫ���Ըı䣬���� ���� ���� ɫ�������ɣ���֤���������� ������ ���� |
| ����3����������2�еģ�2�������й�Һ���룬������ˮϴ�ӹ�����ϴ��Һ��ɫ��ȡ�����������Թ��У��μӹ��� HCl HCl �����ã�ȡ�ϲ���Һ���μ�����KSCN KSCN �������μ�H2O2 H2O2 �� |
��ϲ���2�еģ�2���� ��1������Һ������ɫ������� 1 1 ������2������Һ��Ѫ��ɫ������� 3 3 ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ע�⣺���������������Ч��ԭ��������������Ķ�����Ԫ��a��b��c��d��e�У�a������������Ϊ���������Ķ�����b��d��A2B���⻯���ΪV�η��ӣ�c��+1�����ӱ�e��-1��������8�����ӡ�
�ش��������⣺
��1�� Ԫ��aΪ________��cΪ_______
��2�� ����ЩԪ���γɵ�˫ԭ�ӷ���Ϊ__________________��
��3�� ����ЩԪ���γɵ���ԭ�ӷ����У����ӵĿռ�ṹ����ֱ���ε���_______����ֱ���ε���_______________________����д2�֣�
��4�� ��ЩԪ�صĵ��ʻ��������γɵ�AB�ͻ������У��侧����������ԭ�Ӿ������_______�����Ӿ������_______�������������_______�����Ӿ������_______����ÿ����һ�֣�
��5�� Ԫ��a��b�γɵ�һ�ֻ�������c��b�γɵ�һ�ֻ�������ķ�Ӧ�����ڷ�������У��÷�Ӧ�Ļ�ѧ����ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ע�⣺���������������Ч����������ͼ��ʾװ�ã�β�����������ԣ�����ȡһ����̼�������Բⶨijͭ����Ʒ������CuO��ĩ���н���ͭ�ĺ�����
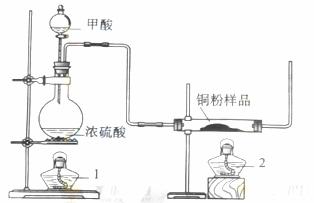
��1���Ʊ�һ����̼�Ļ�ѧ����ʽ�� ��
��2�������У��۲쵽��Ӧ���з���������ʱ ��
β������Ҫ�ɷ��� ��
��3����Ӧ��ɺ���ȷ�IJ���˳��Ϊ ������ĸ��
a.�ر�©������ b.Ϩ��ƾ�1 c.Ϩ��ƾ���2
��4���������г�ȡͭ����Ʒ5.0g����ַ�Ӧ��Ӧ����ʣ����������Ϊ4.8g����ԭ��Ʒ�е���ͭ����������Ϊ ��
��5����Ũ���ᡢŨ���ᡢ����ˮ��˫��ˮ��ѡ�ú��ʵ��Լ������һ���ⶨ��Ʒ�н���ͭ���������ķ�����
����Ʒ�������Ҫ�����ǣ����������������̵�ϸ�ڣ� ��
��д���йط�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ע�⣺���������������Ч��
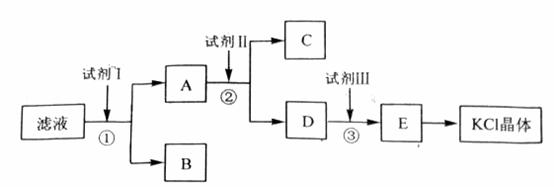
�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ڽ���Һ����ͼ��ʾ������в�����
�ش��������⣺
��1�� ��ʼ��Һ��pH_____________7������ڡ�����С�ڡ����ڡ�������ԭ����_________________________________________________��
��2�� �Լ�I�Ļ�ѧʽΪ______________________�����з�����Ӧ�����ӷ���ʽΪ____________________________________________��
��3�� �Լ���Ļ�ѧʽΪ______________________�����м����Լ����Ŀ����__________________________________________________________________��
��4�� �Լ����������______________________�����з�����Ӧ�����ӷ���ʽΪ__________________________________________________________________��
��5�� ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.7759g���ܽ������100mL����ƿ�У�ÿ��ȡ25.00mL��Һ����0.1000mol��L-1������������Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ25.62mL���ò�Ʒ�Ĵ���Ϊ____________________________________________������ʽ����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com