
| ���� | ������ƽ(������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� | 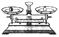 |  |  |  |  | 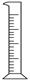 |
| ��� | a | b | c | d | e | f |
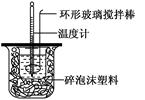
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | ƽ���¶Ȳ� (t2��t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
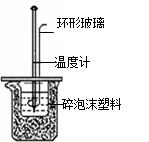
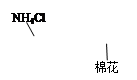  |  |  
|   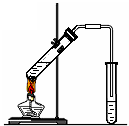  | ||
| A��ʵ������ȡ���� | B������100 mL 0��1 mol /L���� | C���ⶨ�кͷ�Ӧ�ķ�Ӧ�� | D��ʵ�����Ʊ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������к͵ζ�ʱ����10mL��Ͳ��ȡ10.00mL����Һ |
| B����������ƽ����25.20gNaCl���� |
| C�����������£��ù㷺pH��ֽ��ô�ˮ��pHΪ7.0 |
| D����500mL����ƿ����480mL0.100mol/LNaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȼ�ŵľƾ���ȥ��ȼ��һֻ�ƾ��� |
| B����ˮ������ʵ��ʱ��Ҫ����ƿ�ڼӼ�����ʯ�Է�ֹ���� |
| C��������Ũ��������Ƥ����Ҫ������NaOH��Һ��ϴ |
| D��ʢ��������Լ�ƿ��Ҫ������ͼ�ı�־ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
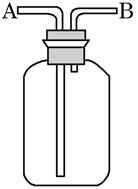
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ�����ģ�ʡ�Լг�װ�ã� | ��Ӧʵ�� |
| A | �Թܡ���ͷ�ι� | ��ϡ���ᡢNa2CO3��Һ�Ƚ�������̼�������ǿ�� |
| B | �ձ�������������ͷ�ιܡ���ֽ | �������ȥ���ᱵ�е�����̼�ᱵ |
| C | �ձ�������������ͷ�ιܡ�����ƿ | �ù����Ȼ�������0.5mol/L����Һ |
| D | �ձ����������������� | ����ͭ��Һ��Ũ���ᾧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ܢݢޢߢ� | B���٢ڢܢ� | C���٢ܢݢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com