
| ʵ�������� | ����Һ���mL | ����ı�Һ�����mL�� |
| 1 | 25.00 | 28.95 |
| 2 | 25.00 | 25.05 |
| 3 | 25.00 | 24.95 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ��������ѧ2012�������ѧ�����п��Ի�ѧ���� ���ͣ�013
|
�����Ȼ�ѧ����ʽ�����ӷ���ʽ�У���ȷ���ǣ� | |
| [����] | |
A�� |
����ı�ȼ���Ȧ�HΪ��890.3 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)��2O2(g)��CO2(g)��2H2O(g)����H����890.3 kJ��mol��1 |
B�� |
500�桢30 MPa�£���0.5 mol��N2(g)��1.5 mol��H2(g)�����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪ��N2(g)��3H2(g) |
C�� |
��������Һ�м������������������Һ��Al3+��2SO |
D�� |
��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ� 2MnO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ����һ��2012�������ѧһ�ָ���ѵ������11�¡���4����ȩ�����ᡡ��(³�ư�) ���ͣ�013
|
����ȵķ������пɷ���õ����½ṹ�Ļ����
�����Լ��� ������KMnO4��Һ�� ��H2/Ni�� ��Ag(NH3)2OH�� ������Cu(OH)2����Һ�� ����û����������й����Ŷ�������Ӧ���Լ��� | |
| [����] | |
A�� |
�٢� |
B�� |
�ڢ� |
C�� |
�ۢ� |
D�� |
�٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ������ѧ2012������ڶ����ۺϿ��Ի�ѧ���� ���ͣ�013
|
�����Ȼ�ѧ����ʽ�����ӷ���ʽ�У���ȷ���� | |
| [����] | |
A�� |
�����ȼ����Ϊ890.3kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ�� CH4(g)��2O2(g)��CO2(g)��2H2O(g)����H����890.3kJ��mol��1 |
B�� |
500�桢30MPa�£���0.5 mol��N2��1.5 mol��H2�����ܱյ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪ�� |
C�� |
��������Һ�м������������������Һ��Al3+��2SO42����2Ba2+��4OH����2BaSO4��AlO2����2H2O |
D�� |
��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO4����6H+��5H2O2��2Mn2+��5O2����8H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ ���ͣ���ѡ��
 2NH3��g�� ��H= -38��6kJ/mol
2NH3��g�� ��H= -38��6kJ/mol 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
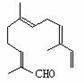
����ȵķ������пɷ���õ����ҽṹ�Ļ�����
�����Լ���������KMnO4��Һ����H2/Ni����Ag(NH3)2OH��Һ��������Cu(OH)2����Һ������û����������й����Ŷ�������Ӧ���Լ���
A���٢ڡ�����B���ڢۡ�����C���ۢܡ���������D���٢�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com