
ͼI ��NO2(g) +CO(g�� CO2(g)+NO(g)��Ӧ�����������仯��ʾ��ͼ��һ�������£��ڹ̶��ݻ����ܱ������и÷�Ӧ�ﵽƽ��״̬�����ı�����һ������X, Y��X�ı仯��ϵ������ͼII��ʾ��
CO2(g)+NO(g)��Ӧ�����������仯��ʾ��ͼ��һ�������£��ڹ̶��ݻ����ܱ������и÷�Ӧ�ﵽƽ��״̬�����ı�����һ������X, Y��X�ı仯��ϵ������ͼII��ʾ��
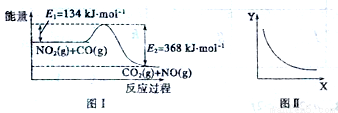
�����й�˵����ȷ���ǣ� ��
A��һ�������£����ܱ������м���1molNO2(g����1molCO(g����Ӧ�ų�234kJ����
B����X��ʾCO����ʼŨ�ȣ���Y��ʾ�Ŀ�����NO2��ת����
C����X��ʾ��Ӧʱ�䣬��Y��ʾ�Ŀ����ǻ��������ܶ�
D����X��ʾ�¶ȣ���Y��ʾ�Ŀ�����CO2�����ʵ���Ũ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����ϵ����������ѧ�Ծ��������棩 ���ͣ������
[��ѧ��ѡ��3�����ʽṹ������]
��1��д��Fe2��������ܲ�ĵ����Ų�ʽ��_____________����Fe2O3��KNO3��KOH��ϼ��ȹ��ڿ���ȡ��ɫ��ˮ��K2FeO4������KNO3����ԭΪKNO2��д���÷�Ӧ�Ļ�ѧ����ʽ_____________��
��2��[Cr��H2O��4Cl2]Cl��2H2O��Cr����λ��Ϊ__________����֪CrO5��CrΪ��6�ۣ���CrO5�ĽṹʽΪ____________��
��3������������CO�����������ȣ�������ɫ�ӷ���Һ��Ni��CO��n�������ʵ�����ԭ�Ӽ۵������������ṩ��������֮��Ϊ18����n��_____________��
��4�����ɰ��SiC���ṹ����ʯ�ṹ���ƣ��������ʯ������һ���Cԭ�ӻ���Siԭ����ͬ��ԭ�Ӳ��ɼ�����õ����ɰ��SiC���ṹ��
��SiC��_________���壬������______________��
�����������һ����ԭ��Ϊ���ģ���SiC�����й�ԭ�����������̼ԭ�ӵ��������Ϊd�������ԭ�Ӵν��ĵڶ�����_________��ԭ�ӣ�������ԭ�ӵľ�����_________��
��5��ͭ�ǵ�����������Ҫ�Ĺ���Ԫ��֮һ���䵥�ʼ���������й㷺��;��CuH�ľ���ṹ����ͼ��ʾ����CuH���ܶ�Ϊdg��cm��3�������ӵ�������ֵΪNA����þ����ı߳�Ϊ ___cm���ú�d��NA��ʽ�ӱ�ʾ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶��ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ����ʾ�����綯��������Ϊ���ݽ�ͷ��������Ӱ��������﮵���Ǻ����д��綯���������õĵ�أ���Ҫ�ӷϾ�������﮵���л���Fe��Al��Li�����ʣ�������ͼ����ʾ��������֪������ ��﮵���ܽ����к��С������������Ӽ������������Һc�к��д������ӣ���������ˮ����������ˮ��
��﮵���ܽ����к��С������������Ӽ������������Һc�к��д������ӣ���������ˮ����������ˮ��


����˵������ȷ���ǣ� ��
A.����Һa�м���������Һ�����Եõ���ɫ����
B.��Һb�м��백ˮ��Ŀ����ʹ����������cΪ���ɫ
C.Ҫ��Li����Һ��������������Һc�м���������Һ������Ũ������ȴ�ᾧ
D.ͼ�еİ�ˮ����������NaOH��Һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫ʡ��һ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
������������ȷ����( )
A������Ʒ�����п������ǿ�俹��ʴ��
B���ں������������п�鱣����Dz��ܸ�ʴ�Dz�������������������������
C��������(����)���Ʋ㱻�ƻ�������������ױ���ʴ
D��������ӵ��������������������Խ�ˮ�µĸ�բ�����Դ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶��ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ������
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��

��1����95��ʱ��ˮ�ĵ���ƽ������ӦΪB����˵������______________________��
25��ʱ����pH=9��NaOH��Һ��pH=4��������Һ��ϣ����û����Һ��pH=7����NaOH��Һ��������Һ�������Ϊ______________��
��2��95��ʱ����100���pH=a��ijǿ����Һ��1���pH=b��ijǿ����Һ��Ϻ���Һ�����ԣ���a��b֮��Ӧ����Ĺ�ϵ��______________��
��3������A����Ӧ���¶��£�pH=2��HCl��Һ��pH=11��ijBOH��Һ�У���ˮ�ĵ���̶ȷֱ��æ�1����2��ʾ�����1______________��2(����ڡ�����С�ڡ��������ڡ�����ȷ����)��
��4������B��Ӧ�¶��£���0.02mol/LBa(OH)2��Һ������ʵ���Ũ�ȵ�NaHSO4��Һ�������Ϻ����Һ��pH=________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶��ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ں���ʱ��һ�̶��ݻ��������ڷ������·�Ӧ��N2O4(g) 2NO2(g)�ﵽƽ��ʱ������������ͨ��һ������N2O4(g)�����´ﵽƽ������һ��ƽ��ʱ��ȣ�N2O4����������� ��
2NO2(g)�ﵽƽ��ʱ������������ͨ��һ������N2O4(g)�����´ﵽƽ������һ��ƽ��ʱ��ȣ�N2O4����������� ��
A������ B������ C����С D�����ж�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶��ϵڶ����¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������Һһ�������Ե��ǣ���
�� pH<7����Һ �� c(H+)=c(OH-)����Һ
��c(H+)=l��10-6mol/L����Һ ��c(H+)>c(OH-)����Һ
�� 25����pH=6����Һ ����ʹ��̪��Һ����ɫ����Һ
A���٢ۢܢݢ� B���ڢܢݢ� C���ݢ� D���ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ��һ����ѧ���Ի�ѧ�Ծ��������棩 ���ͣ������
��ͼ��ʾ��һ�ܱ���������Ħ�����ɻ�����������a��b�ֳɼס������ҡ���״���£��������г���NH30.4mol�������г���HCl��N2�Ļ�����壬��ֹʱ����λ����ͼ��ʾ����֪�ס������������������֮��Ϊ17.3g��

��ش��������⣺
��1�����������������Ϊ ��
��2��������HCl��N2�����ʵ���֮��Ϊ__________��
��3��������aȥ������HCl��NH3��ַ�Ӧ����NH4Cl����������˷�Ӧ��������b��λ�ڿ̶ȡ�______�����������֣������ǹ������ʲ�����ѹǿ������ʱ��ϵ��ƽ��Ħ������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʡ�߶����ڳ�����ѧ���������棩 ���ͣ�ѡ����
����˵����ȷ����

A��ͼ���С�H2=��H1+��H3
B��ͼ���ڴ��������£���Ӧ�Ļ�ܵ���E1+E2
C��ͼ�۱�ʾ������Һ�ζ� NaOH �Ͱ�ˮ�����Һ�ĵ絼�ʱ仯�� ��
��
D��ͼ�ܿɱ�ʾ��CO(g)����CO2(g)�Ĺ�����Ҫ�ų�566kJ ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com