

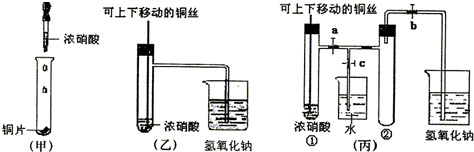
(1)д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
____________________________________________________________________��
(2)�ͼ�װ����ȣ���װ�õ��ŵ���
____________________________________________________________________��
(3)Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ�ã���ʵ��ʱ�ȹرյ��ɼ�___________���ٴ��ɼ�___________������ʹNO2����������Թܡ�
(4)������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β�����______________________��
(5)���Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��___________(�����������״������)��
(1)Cu+4HNO3(Ũ)![]() Cu(NO3)2+2NO2��+2H2O
Cu(NO3)2+2NO2��+2H2O
(2)�ٿ��Կ��Ʒ�Ӧ��������NO2���壬��ֹ��Ⱦ����
(3)c a��b
(4)�ȹر�b���ٹر�a��Ȼ���c��������ס(��ˮ����ë��������)�Թܢ�(�����𰸾���)
(5)1/22.4 mol��L-1��0.045 mol��L-1
������(2)�ҿ���ͨ�����³鶯ͭ˿������HNO3�ķ�Ӧ���ʣ��Ҳ���NO2�ɱ�NaOH����.
(4)�У�ʹ�ձ��е�H2O������Թ��еIJ��������Խ����Ȫʵ��IJ���ԭ�������������������ܷ⣬���ȼ���.


 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)�ס��ҡ�������װ���й�ͬ�����Ļ�ѧ����ʽ��________________��?
(2)�ͼ�װ����ȣ���װ�õ��ŵ��Ǣ�________________����________________��?
(3)Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ�á�ʵ��ʱ�ȹرջ���________���ٴ���_______������ʹNO2����������Թܣ�������������Թܺ�ͭ˿��������Һ���롣��ʹ�ձ��е�ˮ������Թܣ�Ӧ��β���?________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
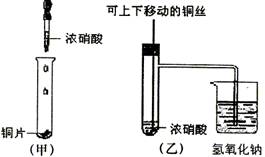
(10��) ��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�
��1�� д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ ��
��2���ͼ�װ����ȣ���װ�õ��ŵ���
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ��,��ʵ��ʱ�ȹرյ��ɼ�
���ٴ��ɼ� ������ʹNO2����������Թܡ�
��4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β�
�� ��
��5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��
�������������״�����㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09��10��ɶ�ʯ����ѧ��һ������ĩ���Ի�ѧ�� ���ͣ�ʵ����
(10��)��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�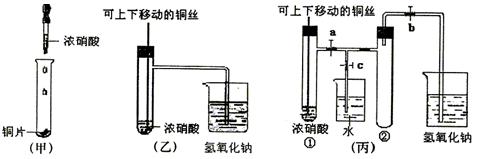
��1�� д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ ��
��2���ͼ�װ����ȣ���װ�õ��ŵ���
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ��,��ʵ��ʱ�ȹرյ��ɼ�
���ٴ��ɼ� ������ʹNO2����������Թܡ�


��4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β�
�� ��
��5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��
�������������״�����㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��8�֣���ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ���Ľ����װ�ã�
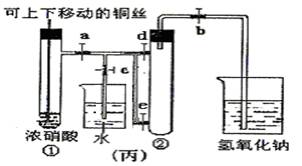
��1��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ ��
��2���ͼ�װ����ȣ���װ�õ��ŵ� ��
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ�á��ȹرյ��ɼ� ���ٴ��ɼ� ������ʹNO2����������Թܡ�
��4��������������Թܺ���ʹ�ձ��е�ˮ������Թ�Ӧ��β��� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com