
����ʵ���������������ʵ��������˵����ȷ����(����)
A�������²ⶨ������Ħ���������22.4 L��mol��1
B��100 mL 0.1 mol��L��1 NaOH��Һ��100 mL 0.1 mol��L��1CH3COOH��Һ��Ӧ�ų�����������573 J
C������1.0 mol��L��1 NaCl��Һ����ʱ��������ƿ�Ŀ̶��ߵ���������ҺŨ��ƫ��
D���к͵ζ�ʱ����ƿ����ˮ��ע�����Һ���������ҺŨ�ȼ�С

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڱ�״���£���V L A����(Ħ������M g/mol)����100mlˮ��(A��ˮ����Ӧ)������A��ˮ��Һ�ܶ�Ϊ�� g/cm3�������Һ�����ʵ���Ũ��(mol/L)Ϊ( )
A��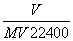 B��
B�� 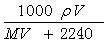 C��
C�� D��
D��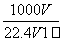
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2�ȱ�����ȡ������1��2������(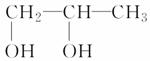 )ʱ����Ҫ���������ļ�����Ӧ
)ʱ����Ҫ���������ļ�����Ӧ
A���ӳɡ���ȥ��ȡ������ B����ȥ���ӳɡ�ˮ��
C��ȡ������ȥ���ӳ� D����ȥ���ӳɡ���ȥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�� ѧ��ȤС��Ϊ��̽��ijЩ��������ʣ��������ͼ��ʾ��ʵ��װ�á�ʵ��ʱ��A��D�в���������ͬʱͨ��C�С�(KΪֹˮ�У����ּг���������ȥ)
ѧ��ȤС��Ϊ��̽��ijЩ��������ʣ��������ͼ��ʾ��ʵ��װ�á�ʵ��ʱ��A��D�в���������ͬʱͨ��C�С�(KΪֹˮ�У����ּг���������ȥ)
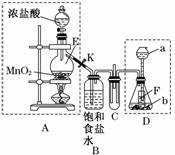
�ش��������⣺
(1)����ʵ��ǰ���A����װ�õ������Եķ���_____________________ ___
___
________________________________________________________________________��
(2)����E��������_______________________����ƿ��С�Թ�F��������________________________________________________________________________��
(3)��b����ʯ�ң�a��________ʱ����C���а��̲�����д�����ɰ��̵Ļ�ѧ����ʽ________________________________________________________________________
________________________________________________________________________��
(4)��a����������Ϊ75%�����ᣬb���������Ʒ�ĩ����C��ʢ������BaCl2��Һʱ��д��C�з�����Ӧ�����ӷ���ʽ_________________________________________��
(5)�ӻ����ĽǶȳ���������ʵ��װ������Ҫ�Ľ�����___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס�������ͬѧͬʱ�����һ�ݱ�����������к͵ζ�ʵ������ݼ�¼�ʹ�������ͬѧ��Ƶı����ʵ���¼������£�
| ���� | V(NaOH)/mL | ��V(HCl)/mL |
| 1 | 20.48 | 22.46 |
| 2 | 25.00 | 21.98 |
| 3 | 25.00 | 22.36 |
��ͬѧ��Ƶı����ʵ���¼������£�
| ���� | V(NaOH)/mL | ��V(NaOH) /mL | V(HCl)/mL | ��V(HCl) /mL | ||
| ʼ���� | �ն��� | ʼ���� | �ն��� | |||
| 1 | 0.10 | 25.00 | 0.00 | 24.80 | ||
| 2 | 0.00 | 24.80 | 0.10 | 22.32 | ||
| 3 | 0.00 | 24.80 | 0.00 | 24.70 |
��ش��������⣺
(1)����Ƚϼס��������ͼ�¼���������ͬѧ�ı����ʵ���¼������֮����ԭ����_____________________________________________��
(2)������ͬѧ��ʵ�����ݣ���c(HCl)��0.10 mol��L��1�����õ�c(NaOH)��________ mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ��� ��
��A��t��ʱ��ij��Һ��pH��6�������Һһ��Ϊ����
��B�������£���pH��11�İ�ˮϡ�ͺ���Һ���������ӵ�Ũ�Ⱦ�����
��C�������£���pH��11��NaOH��Һ��pH��3��CH3COOH��Һ�������Ϻ���Һ��pH��7
��D�������£����ʵ���Ũ�Ⱥ������ͬ��K2CO3��K2SO4��HC1��Һ��Ϻ���Һ��
pH��7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��������ȡCH3COCOOCH2CH2CH3�����������Ҫ�����з�Ӧ��˳����
a.���� b.��ԭ c.ȡ�� d.�ӳ� e.��ȥ f.�к� g.���� h.����
A. e��d��c��a��h B. b��d��f��g��h
C. a��e��d��c��h D. b��a��e��c��f
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�鷽����ʵ����۲����ϵ���(����)

A����֤���ǽ�����S>C>Si
B�������Zn��Cuԭ���
C��ʵ������ȡCl2
D����ɫ����ΪBaSO4
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com