
| Ԫ�� | Mn | F | |
| ������ ��kJ?mol-1�� | I1 | 717 | 759 |
| I2 | 1509 | 1561 | |
| I3 | a | b | |
| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2C2 | 272 | 423 | ������Ȼ��� |
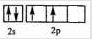 ��
�� ��ԭ������AС��C����AΪCԪ�أ�CΪOԪ�أ���BΪNԪ�أ�D�Ķ�����������OԪ�ص������Ӿ�����ͬ�ĵ��Ӳ�ṹ��DӦΪMgԪ�أ�D��Eͬ���壬��EӦΪCaԪ�أ�F3+����M��3d�������Ϊ����״̬�������ӵĺ��������Ϊ23��F��ԭ������Ϊ26��ӦΪFeԪ�أ����Ԫ�ض�Ӧԭ�ӵĽṹ�����ʼ�����������ʽ����⣮
��ԭ������AС��C����AΪCԪ�أ�CΪOԪ�أ���BΪNԪ�أ�D�Ķ�����������OԪ�ص������Ӿ�����ͬ�ĵ��Ӳ�ṹ��DӦΪMgԪ�أ�D��Eͬ���壬��EӦΪCaԪ�أ�F3+����M��3d�������Ϊ����״̬�������ӵĺ��������Ϊ23��F��ԭ������Ϊ26��ӦΪFeԪ�أ����Ԫ�ض�Ӧԭ�ӵĽṹ�����ʼ�����������ʽ����⣮ ��
�� ��ԭ������AС��C����AΪCԪ�أ�CΪOԪ�أ���BΪNԪ�أ�D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��DӦΪMgԪ�أ�D��Eͬ���壬��EӦΪCaԪ�أ�F3+����M��3d�������Ϊ����״̬�������ӵĺ��������Ϊ23��F��ԭ������Ϊ26��ӦΪFeԪ�أ�
��ԭ������AС��C����AΪCԪ�أ�CΪOԪ�أ���BΪNԪ�أ�D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��DӦΪMgԪ�أ�D��Eͬ���壬��EӦΪCaԪ�أ�F3+����M��3d�������Ϊ����״̬�������ӵĺ��������Ϊ23��F��ԭ������Ϊ26��ӦΪFeԪ�أ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ��Ҫ�� | ѡ�� |
| ��1����ȥʳ��������ɳ�ӣ�SiO2�� | |
| ��2��֤��Na2CO3��Һ�ʼ��� | |
| ��3����ȥNa2CO3������������NaHCO3 | |
| ��4������NaNO3��KNO3 | |
| ��5����ȥ���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
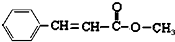
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���٢ۢ� |
| C���ڢݢ� | D���٢ܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٣��ڣ��� |
| B���ۣ��ڣ��� |
| C���ڣ��٣��� |
| D���ۣ��٣��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ĵ�����������٣��ڣ��� |
| B���Ի�����ɵ�Σ��������С |
| C����ͭ��Ũ����������죬����ȡ����ͭ����ѷ����Ǣ� |
| D������;�������ĵ�ͭ����������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com