
��֪ú�Ľ����ṹģ����ͼ��ʾ��
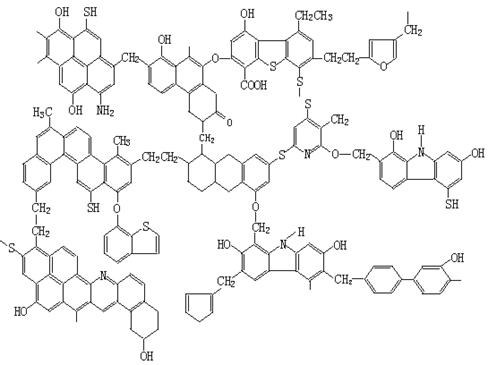
��1����ú�Ľṹģ��������ú�ǹ�ҵ�ϻ��___________������Ҫ��Դ��
��2���ҹ���Լ70%��ú��ֱ������ȼ�յģ���ú�Ľṹģ�����������ṩ������ͬʱ����������___________���������ʣ�������صĴ�����Ⱦ��
��3������ú�������������Լ���87%�ķ����ŷ������̳��ŷ���Ҳ�ɼ���80%���°��ﱽ���ۦ����ŵ��ŷ���Ҳ���٣�ͬʱ��ú20%��30%������ú��������ԭ�������ù������ȼ�չ����������ȶ��������Σ����������������������������___________��
A���Ȼ��� B�������� C������� D����������
��4��ij����ú������������ʯ��ʯ������������û�ѧ����ʽ��ʾ�䡰�����̣�
��5���������ġ���ú�������⣬Ϊ�˽��úȼ������ɵ���Ⱦ��������ú�����ü�ֵ��ú��Դ���ۺ����÷�������___________��___________��___________�ȣ�
��1�����㣨2�����������������3��BD
��4��CaCO3![]() CaO+CO2�� 2CaO+2SO2+O2
CaO+CO2�� 2CaO+2SO2+O2![]() 2CaSO4
2CaSO4
��5��ú�ĸ���������Һ��
��1��ú�ṹ�к��н϶�ı���������������ú��ǿ�ȿ���ʹ���ֽ⣬�Ӷ���÷�������
��2��ú�ṹ�к���S��N������ֱ��ȼú�ܲ���SO2�͵��������Ⱦ������
��3��úȼ�ղ���SO2�����������ʹ�������ȶ��������Σ����Թ������ѡ�������ƻ��������ƣ�����ӦΪ��SO2+CaO![]() CaSO3 2CaSO3+O2
CaSO3 2CaSO3+O2![]() 2CaSO4
2CaSO4
Ca(OH)2+SO2=CaSO3+H2O 2CaSO3+O2![]() 2CaSO4
2CaSO4
��4��������ʯ��ʯ��������������ķ�Ӧ�ɱ�ʾΪ��CaCO3![]() CaO+CO2��
CaO+CO2��
2CaO+2SO2+O2![]() 2CaSO4
2CaSO4
��5���������DZ���ѡѧ��Ҫ����֮һ��ú�ĸ���������Һ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�ش��������⣺
(1)��ú�Ľṹģ��������ú�ǹ�ҵ�ϻ��__________������Ҫ��Դ��
(2)�ҹ���Լ70%��ú��ֱ������ȼ�յġ���ú�Ľṹģ�����������ṩ������ͬʱ����������__________��__________���������ʣ�������صĴ�����Ⱦ��
(3)����ú�������������Լ���87%�ķ����ŷ������̳��ŷ���Ҳ�ɼ���80%���°��ﱽ����a���ŵ��ŷ���Ҳ���٣�ͬʱ��ú20%��30%������ú��������ԭ�������ù������ȼ�չ����������ȶ��������Ρ�ij����ú������������ʯ��ʯ������������û�ѧ����ʽ��ʾ�䡰�����̣�_______________________��________________________________��
(4)Ϊ�˽��úȼ������ɵ���Ⱦ��������ú�����ü�ֵ��ú��Դ���ۺ����÷�������__________��__________��__________�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1����ú�Ľṹģ��������ú�ǹ�ҵ�ϻ��_____________������Ҫ��Դ��
��2���ҹ���Լ70%��ú��ֱ������ȼ�յģ���ú�Ľṹģ�����������ṩ������ͬʱ����������____________���������ʣ�������صĴ�����Ⱦ��
��3������ú�������������Լ���87%�ķ����ŷ������̳��ŷ���Ҳ�ɼ���80%���°��ﱽ���ۦ����ŵ��ŷ���Ҳ���٣�ͬʱ��ú20%��30%������ú��������ԭ�������ù������ȼ�չ����������ȶ��������Ρ����������������������������_____________��
A.�Ȼ��� B.������ C.����� D.��������
��4��ij����ú������������ʯ��ʯ������������û�ѧ����ʽ��ʾ�䡰�����̡�
��5���������ġ���ú�������⣬Ϊ�˽��úȼ������ɵ���Ⱦ��������ú�����ü�ֵ��ú��Դ���ۺ����÷�������_____________��_____________��_____________�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)��ú�Ľṹģ��������ú�ǹ�ҵ�ϻ��__________������Ҫ��Դ��
(2)�ҹ���Լ70%��ú��ֱ������ȼ�յģ���ú�Ľṹģ�����������ṩ������ͬʱ����������__________���������ʣ�������صĴ�����Ⱦ��
(3)����ú�������������Լ���87%�ķ����ŷ������̳��ŷ���Ҳ�ɼ���80%���°��ﱽ��(a)�ŵ��ŷ���Ҳ���٣�ͬʱ��ú20%��30%������ú��������ԭ�������ù������ȼ�Ϲ����������ȶ��������Ρ����������������������������__________��
A.�Ȼ��� B.������ C.����� D.��������
(4)�������ġ���ú�������⣬Ϊ�˽��úȼ������ɵ���Ⱦ��������ú�����ü�ֵ��ú��Դ���ۺ����÷�������__________��__________��__________�ȡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com