
| ФӘЛШ | ФӘЛШРФЦК»тФӯЧУҪб№№РЕПў |
| Q | ФӯЧУәЛНвУР6ЦЦІ»Н¬ФЛ¶ҜЧҙМ¬өДөзЧУ |
| R | ЧоНвІгөзЧУКэКЗҙОНвІгөзЧУКэөД3ұ¶ |
| X | ЖшМ¬Зв»ҜОпөДЛ®ИЬТәіКИхјоРФ |
| Y | өЪИэЦЬЖЪФӘЛШөДјтөҘАлЧУЦРАлЧУ°лҫ¶ЧоРЎ |
| Z | өҘЦКОӘТш°ЧЙ«№ММеЈ¬ФЪҝХЖшЦРИјЙХ·ўіц»ЖЙ«»рСж |

| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәөҘСЎМв

AЈ®ФЪЦЬЖЪұнЦРЈ¬ЧеРтКэ¶јөИУЪёГФӘЛШөДЧоНвІгөзЧУКэЈ® |
BЈ®·ЗҪрКфРФЧоЗҝөДФӘЛШЈ¬ЖдЗв»ҜОпИЬУЪЛ®өДЛбРФЧоЗҝ |
CЈ®јоҪрКфЛжФӯЧУРтКэФцҙуЈ¬ИЫөгҪөөНЈ»ВұЧеФӘЛШөДөҘЦКЈ¬ЛжФӯЧУРтКэФцҙуЈ¬ИЫөгТАҙОЙэёЯЈ® |
| DЈ®Н¬Т»ЦЬЖЪөДЦчЧеФӘЛШЈ¬ҙУЧуЦБУТЈ¬ФӯЧУ°лҫ¶јхРЎЈ¬ЛьГЗЛщРОіЙөДјтөҘАлЧУ°лҫ¶ҙУЧуЦБУТТАҙОФцҙу |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәМоҝХМв
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәөҘСЎМв
| AЈ®dЈјcЈјaЈјb | BЈ®cЈјdЈјbЈјa | CЈ®dЈјaЈјbЈјc | DЈ®cЈјdЈјaЈјb |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәөҘСЎМв
| AЈ®OЈә1s22s22p4 | BЈ®PЈә1s22s22p63s23p3 |
| CЈ®CrЈә1s22s22p63s23p63d54s1 | DЈ®MnЈә1s22s22p63s23p63d54s2 |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәөҘСЎМв
AЈ®МјФӯЧУөД№мөАұнКҫКҪЈә | BЈ®ҙОВИЛб·ЦЧУөДөзЧУКҪЈә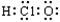 |
CЈ®CH4SiөДҪб№№КҪЈә | DЈ®ЦКБҝКэОӘ137өДұөФӯЧУЈә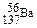 |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәөҘСЎМв
| AЈ®CsЈ«ЎўKЈ«ЎўMgЈ«ЎўAl3Ј« | BЈ®FЎўFЁDЎўClЁDЎўBrЁD |
| CЈ®Ca2Ј«ЎўKЈ«ЎўClЁDЎўO2ЁD | DЈ®AlЎўAl3Ј«ЎўMgЎўK |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәөҘСЎМв
| AЈ®Н¬ЦЬЖЪФӘЛШЦРXөДЧоёЯјЫСх»ҜОп¶ФУҰЛ®»ҜОпөДјоРФЧоЗҝ |
| BЈ®ФӯЧУ°лҫ¶XЈҫYЈ¬АлЧУ°лҫ¶XЈ«ЈҫZ2Јӯ |
| CЈ®Н¬ЦЬЖЪФӘЛШЦРYөДЖшМ¬Зв»ҜОпОИ¶ЁРФЧоЗҝ |
| DЈ®ИэЦРФӘЛШТ»¶ЁКфУЪ¶МЦЬЖЪФӘЛШ |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәІ»Пк МвРНЈәөҘСЎМв
| AЈ®HClO4ЎўH2SO4ЎўH3PO4өДЛбРФТАҙОФцЗҝ | BЈ®HClЎўHBrЎўHIөДОИ¶ЁРФТАҙОФцЗҝ |
| CЈ®ДЖЎўГҫЎўВБөД»№ФӯРФТАҙОјхИх | DЈ®NЎўOЎўFФӯЧУ°лҫ¶ЦрҪҘФцҙу |
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com