
��. ��ˮ��Һ�гȺ�ɫ��Cr2O72-���ɫ��CrO42-������ƽ���ϵ��
Cr2O72-��H2O  2CrO42-��2H+����K2Cr2O7����ˮ���ϡ��Һ�dz�ɫ��
2CrO42-��2H+����K2Cr2O7����ˮ���ϡ��Һ�dz�ɫ��
��1����������Һ�м���NaOH��Һ����Һ�� ɫ����Ϊ ��
��2�����Ѽ���NaOH��Һ�ģ�1�����ټ������ϡH2SO4������Һ�� ɫ����Ϊ ��
��3����ԭ��Һ�м���Ba(NO3)2��Һ����֪BaCrO4Ϊ��ɫ��������ƽ���� �����ƶ�����Һ��ɫ�� ��������������dz�����䡱��
��.ʵ������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飺
1.����100mL 0.10mol/L NaOH����Һ��
2.ȡ20.00mL����ϡ������Һ������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶı�NaOH��Һ���еζ���
3.�ظ������ζ�����2��3�Σ���¼�������¡�
|
ʵ���� |
NaOH��Һ��Ũ�� ��mol/L�� |
�ζ����ʱ��NaOH��Һ����������mL�� |
����������Һ����� ��mL�� |
|
1 |
0.10 |
22.62 |
20.00 |
|
2 |
0.10 |
22.58 |
20.00 |
|
3 |
0.10 |
22.60 |
20.00 |
��1���ζ��ﵽ�յ�������� ����ʱ��ƿ����Һ��pH��ΧΪ ��
��2�������������ݣ��ɼ�����������Ũ��ԼΪ ��
��3����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ �IJ�����Ȼ��ѹ������ʹ���첿�ֳ�����Һ��



�� �� ��
��4��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ��� ����ѡ�۷֣���
A���ζ��յ����ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ
C����ƿˮϴ��δ����
D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ��
E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶���
F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
��.ÿ��1�֣�1���ƣ��к�H����ʹc(H+)��С��ƽ��������Ӧ�����ƶ�
��2���Ⱥ�ɫ������������c(H+)��ƽ�����淴Ӧ�����ƶ� ��3������Ӧ���ң� ��dz
��.��1�������һ������������Һ���룬��Һ����ɫǡ�ñ�dz��ɫ���Ұ�����ڲ���ɫ ��8.2��10 ��2��0.1130mol/L����д��λ��1�֣� ��3���� ��4��EF��2�֣�
��������
�����������.��1����������Һ�м���NaOH��Һ����Һ��OH��Ũ�������к������ӣ����������ӵ�Ũ�ȡ������Cr2O72-��H2O  2CrO42-��2H+��֪��ƽ��������Ӧ�����ƶ���������Һ�ʻ�ɫ��
2CrO42-��2H+��֪��ƽ��������Ӧ�����ƶ���������Һ�ʻ�ɫ��
��2�����Ѽ���NaOH��Һ�ģ�1�����ټ������ϡH2SO4������������c(H+)��ƽ�����淴Ӧ�����ƶ������Һ�ʳȺ�ɫ��
��3����ԭ��Һ�м���Ba(NO3)2��Һ����Ba2�����CrO42������BaCrO4��ɫ����������CrO42-��Ũ�ȣ�����ƽ��������Ӧ�����ƶ����������ӵ�Ũ�Ƚ��ͣ������Һ��ɫ����dz��
��.��1�������ڼ���ʹ��̪�Ժ�ɫ������ʹ��̪����ɫ������������������Һ�ζ�����ʱ���ζ��ﵽ�յ�����������һ������������Һ���룬��Һ����ɫǡ�ñ�dz��ɫ���Ұ�����ڲ���ɫ�����ڷ�̪�ı�ɫ��Χ��8.2��10.0�����Դ�ʱ��ƿ����Һ��pH��ΧΪ8.2��10.0��
��2�����ݱ������ݿ�֪������ʵ������������������Һ������ֱ���22.62ml��0.10ml��22.52ml��22.58ml��0.10ml��22.48ml��22.60ml��0.10ml��22.50ml����������ʵ����ƽ����������������Һ�������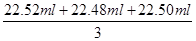 ��22.50ml����������Ũ����
��22.50ml����������Ũ���� ��0.1130mol/L��
��0.1130mol/L��
��3�����ڼ�ʽ�ζ��ܵ������Ǻ��в�������ܣ���˼�ѹ������ʹ���첿�ֳ�����Һ����ȷ������ѡ�����
��4������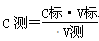 ��֪������C�ꡢV����Ϊ��ֲ������C��Ĵ�Сȡ����V��Ĵ�С����V�꣺ƫ���ƫС����C��ƫ���ƫС���ݴ˿����жϡ�A���ζ��յ����ʱ���Ӷ����������ƫС�������������������Һ�����ƫС���ⶨ���ƫ�ͣ�B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ�������ᱻϡ�ͣ�Ũ�Ƚ��͡������������������Һ�����ƫС���ⶨ���ƫ�ͣ�C����ƿˮϴ��δ������ı����ʵ����ʵ�������Ӱ������D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ�У���ʵ�����������������ҺŨ��ƫ�ߡ�����к�����ʱ��������������Һ�����ƫС���ⶨ���ƫ�ͣ�E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶��ߣ�������Ũ��ƫ�ͣ������к�����ʱ��������������Һ�����ƫ�ⶨ���ƫ�ߣ�F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ����ʵ����������������Һ�����ƫ�ⶨ���ƫ�ߣ����Դ�ѡEF��
��֪������C�ꡢV����Ϊ��ֲ������C��Ĵ�Сȡ����V��Ĵ�С����V�꣺ƫ���ƫС����C��ƫ���ƫС���ݴ˿����жϡ�A���ζ��յ����ʱ���Ӷ����������ƫС�������������������Һ�����ƫС���ⶨ���ƫ�ͣ�B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������Һ��ϴ�������ᱻϡ�ͣ�Ũ�Ƚ��͡������������������Һ�����ƫС���ⶨ���ƫ�ͣ�C����ƿˮϴ��δ������ı����ʵ����ʵ�������Ӱ������D������NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ�У���ʵ�����������������ҺŨ��ƫ�ߡ�����к�����ʱ��������������Һ�����ƫС���ⶨ���ƫ�ͣ�E������NaOH����Һʱ������ʱ��������ƿ�Ŀ̶��ߣ�������Ũ��ƫ�ͣ������к�����ʱ��������������Һ�����ƫ�ⶨ���ƫ�ߣ�F����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ����ʵ����������������Һ�����ƫ�ⶨ���ƫ�ߣ����Դ�ѡEF��
���㣺�������������ƽ��״̬��Ӱ�죻����к͵ζ����������㡢������


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ�߶���ѧ����ĩ��ѧ������⣨���ۣ���ѧ���� ���ͣ�ѡ����
���и�����������ˮ��Һ�д������棬�Ҹ�ˮ��Һ��ʹ������Һ������
A��Fe2+��K+��NO3-��SO42- B��Cu2+��Mg2+��NO3-��SO42-
C��Fe3+��Na+��Cl-��C6H5O- D��Fe3+��K+��I-��Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�����������ˮ��Һ�д������棬�Ҹ�ˮ��Һ��ʹ������Һ������
A��Fe2+��K+��NO3-��SO42- B��Cu2+��Mg2+��NO3-��SO42-
C��Fe3+��Na+��Cl-��C6H5O- D��Fe3+��K+��I-��Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�����������ˮ��Һ�д������棬�Ҹ�ˮ��Һ��ʹ������Һ������
A��Fe2+��K+��NO3-��SO42- B��Cu2+��Mg2+��NO3-��SO42-
C��Fe3+��Na+��Cl-��C6H5O- D��Fe3+��K+��I-��Cl-
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com