��2011?����ģ�⣩[��ѧ-ѡ���л���ѧ����]
�л���AΪ����������ͼ��������Է�������Ϊ70������ط�Ӧ����ͼ��ʾ������B��D��E�Ľṹ�о�����2��-CH
3�����ǵĺ˴Ź��������о�����4���壮

��ش�
��1��D�ķ���ʽΪ
C5H10O
C5H10O
B�����������ŵ�����Ϊ
��ԭ��
��ԭ��
��
��2����ķ�Ӧ����Ϊ
d
d
��ķ�Ӧ����Ϊ
a��b
a��b
������ĸ��ţ���
a����ԭ��Ӧ b���ӳɷ�Ӧ c��������Ӧ d����ȥ��Ӧ
��3��д����Ӧ��Ļ�ѧ����ʽ
CH
3-CH��CH
3��-CH
2-CH
2Br+NaOH
CH
3-CH��CH
3��-CH=CH
2+NaBr+H
2O
CH
3-CH��CH
3��-CH
2-CH
2Br+NaOH
CH
3-CH��CH
3��-CH=CH
2+NaBr+H
2O
��
��4����˳���칹�ҽṹ����2��-CH
3��A��ͬ���칹��Ľṹ��ʽΪ
��
��5��E�ж���ͬ���칹�壬�����ܷ���������Ӧ�����������������������������ܷ�����ȥ��Ӧ����ṹ��ʽΪ
��


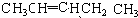
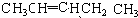


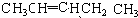 ��
��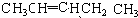 ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

