
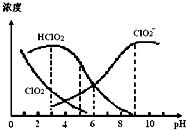
| A�� | ʹ�ø�Ư��������pHԼΪ5.0 | |
| B�� | ���¶���NaClO2��Һ�У�c�� Na+����c��ClO2-����c��OH-����c��H+�� | |
| C�� | ���¶���HClO2�ĵ���ƽ�ⳣ������ֵKa��1.0��10-6 | |
| D�� | ��ͬpH��NaClO2��Һ�д������й�ϵ��c�� Na+��=c��ClO2-��+c��HClO2��+c��ClO2�� |
���� A��HClO2��Ư��֯�����Ч�ɷ֣�ClO2���ж����壬Ӧ����pHֵ��ΧʹƯ����Ư����ǿ����ҪʹClO2Ũ�Ƚ�С��
B������ͼ֪������Һ�ʼ��ԣ���c��OH-����c��H+����������ˮ��̶Ƚ�С����ϵ���غ��жϣ�
C����HClO2�ĵ���ƽ�ⳣ��K=$\frac{c��{H}^{+}����c��Cl{{O}_{2}}^{-}��}{c��HCl{O}_{2}��}$��
D����Һ�п��ܻ�����c��Cl-����
��� �⣺A��HClO2��Ư��֯�����Ч�ɷ֣�ClO2���ж����壬ҪʹƯ����Ư����ǿ����HClO2�ĺ����ߣ���ҪʹClO2Ũ�Ƚ�С������ͼ��֪��pH��С��ClO2�����ϴ�pH����HClO2�ĺ����ϵͣ�����Һ��pHΪ5.0���ң���A��ȷ��
B������ͼ֪������Һ�ʼ��ԣ���c��OH-����c��H+����������ˮ��̶Ƚ�С����ϵ���غ��c�� Na+����c��ClO2-����c��OH-����c��H+������B��ȷ��
C����HClO2�ĵ���ƽ�ⳣ��K=$\frac{c��{H}^{+}����c��Cl{{O}_{2}}^{-}��}{c��HCl{O}_{2}��}$����pH=6ʱ��c��HClO2��=c��ClO2-������K=$\frac{c��{H}^{+}����c��Cl{{O}_{2}}^{-}��}{c��HCl{O}_{2}��}$=c��H+��=1.0��10-6����C��ȷ��
D����Һ�п��ܻ�����c��Cl-�������Կ��ܴ���c�� Na+��=c��ClO2-��+c��HClO2��+c��ClO2��+c��Cl-������D����
��ѡD��
���� ���⿼������Ũ�ȴ�С�Ƚϣ�Ϊ��Ƶ���㣬���ؿ���ѧ��ͼ��������������ʣ���ȷ�������ʼ���Ӧԭ���ǽⱾ��ؼ���ע����Һ�д������ɷ֣��״�ѡ����D��


 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A | CH3CH3+Cl2$\stackrel{��}{��}$CH3CH2Cl+HCl CH2�TCH2+HCl-��CH3CH2Cl | ��Ϊȡ����Ӧ |
| B | �����ȼ�� ��ϩʹ���Ը��������Һ��ɫ | �л���������� ������Ӧ |
| C | Cl2+2Br-�T2Cl-+Br2 Zn+Cu2+�TZn2++Cu | ��Ϊ���ʱ���ԭ�� �û���Ӧ |
| D | 2Na2O2+2H2O�T4NaOH+O2�� Cl2+H2O?HCl+HClO | ��Ϊˮ����ԭ���� ������ԭ��Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | ����������mL�� | �¶� | �������� |
| �� | 2Ml | 20 | �� |
| �� | 2mL | 20 | 10�α���MnS04��Һ |
| �� | 2mL | 30 | �� |
| �� | 1mL | 20 | 1mL����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ��ij��Һ�м�������ϡ�����������ٵμ�BaCl2��Һ | �а�ɫ�������� | ԭ��Һ����SO42- |
| B | Agl�����е���ϡKCl��Һ | �а�ɫ�������� | AgCl��AgI������ |
| C | ��CH3CH2X�м�������AgNO3��Һ������ | ��dz��ɫ�������� | CH3CH2X�к���Br- |
| D | C2H5OH��Ũ����170�湲�ȣ��Ƶõ�����ͨ������KMnO4��Һ | KMnO4��Һ��ɫ | ʹKMnO4��Һ��ɫ������ϩ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��3a+0.5b��mol | B�� | ��3a+0.5b��mol | C�� | ��3a+0.5b+3p��mol | D�� | ��3a+0.5b-3p��mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | MgCl2 | B�� | NH4Cl | C�� | CO2 | D�� | H2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʵ��ʽC2H402 | B�� | �۱�ϩ�Ľṹ��ʽ��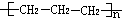 | ||
| C�� | �ǻ��ĵ���ʽ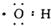 | D�� | ������ӵ����ģ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
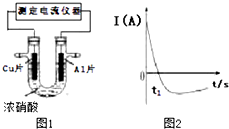 �����£�����ȥ��������Ĥ��Al��CuƬ����ŨHNO3�����ԭ��أ���ͼ1�������ԭ��صĵ���ǿ�ȣ�I����ʱ�䣨t���ı仯��ͼ2��ʾ����֪0-t1ʱ��ԭ��صĸ�����AlƬ����Ӧ�������к���ɫ�������������˵������ȷ���ǣ�������
�����£�����ȥ��������Ĥ��Al��CuƬ����ŨHNO3�����ԭ��أ���ͼ1�������ԭ��صĵ���ǿ�ȣ�I����ʱ�䣨t���ı仯��ͼ2��ʾ����֪0-t1ʱ��ԭ��صĸ�����AlƬ����Ӧ�������к���ɫ�������������˵������ȷ���ǣ�������| A�� | 0-t1ʱ�������ĵ缫��ӦʽΪ��2H++NO3--e-�TNO2+H2O | |
| B�� | 0-t1ʱ����Һ�е�H+��Cu�缫�ƶ� | |
| C�� | t1ʱ�������ĵ缫��ӦʽΪ��Cu-2e-�TCu2+ | |
| D�� | t1ʱ��ԭ����е��������������ı�����ΪAl��Ũ�����жۻ�������Ĥ�谭��Al�Ľ�һ����Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com