
�����£���16 mol N2��24 mol H2�Ļ������ͨ��һ���̶��ݻ�Ϊ4L���ܱ������У��������·�Ӧ��N2��g��+3H2��g��![]() 2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ���� �� ��
2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ���� �� ��
A���ﵽƽ��ʱ��N2��H2��ת����֮��Ϊ1��1
B��10������v(H2)=0.35mol/(L��min)
C��ƽ����������ܶ�Ϊ128g/L
D��ƽ���������У�n��N2����n��H2����n��NH3��=1��3��2

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ���ѡ��
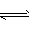 2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ����
2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ���� �鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com