
���� ��1������������Һ��ˮ�����ɽ�������࣬��ˮ������������������ʹ��۳��ﵽ����Ŀ�ģ���ȥ���������������ɱ����
��2������ˮ�dz�ȥ��þ���ӣ�
��3������Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mgCaO����֮�൱�����ʣ���7.1mgMgO������ˮ�е�Ca2+��Mg2+���������CaO����������õ���
��4��Ӳˮ������Ҫ�Ѹ����Ӻ�þ����ȫ�������������������ƺ�þ���ӡ�̼��������ӷ�Ӧ������̼���Ƴ��������ӣ�
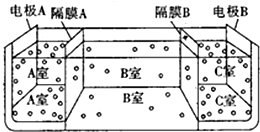
��5���������ӽ���Ĥֻ��������������ͨ���������ӽ���Ĥֻ��������������ͨ�������ص������������ӷŵ磬���������������ӷŵ磻
�ڵ缫AΪ�������缫BΪ���������ص������������ӷŵ磬���������������ӷŵ磮
��� �⣺��1��������������ˮ�е�С�������������۳ɽϴ�Ŀ����������ٳ�������Ҫ���ã�Ҫ�߱�ˮ�����ɽ�������ʣ����Գ������λ�������ˮ�з���ˮ��������Ӧ�������コ�壬��������������ˮ������������������������ӣ���������������������ԣ������ܾ�ˮ��������������������������Ҳ������ˮ�����ã�������������������ʹ��۳��ﵽ��ˮ��Ŀ�ģ�������Һ�л��в���ϸ���ȣ��к����ʣ���Ҫ������ɱ����
�ʴ�Ϊ�������������������������������������λ�������ˮ�з���ˮ�ⷴӦ������Ӧ���������コ�壬����������Ȼˮ�е������ﲢ�ƻ���Ȼˮ�е����������Ľ��壬ʹ��۳��ﵽ��ˮ��Ŀ�ģ�������ɱ����
��2��Ӳˮ������ָ���dz�ȥ�����ˮ�е�Ca2+��Mg2+�ȵĹ��̣��ʴ�Ϊ����ȥ�����ˮ�е�Ca2+��Mg2+�ȵĹ��̣�
��3��ij��Ȼˮ��c��Ca2+��=1.2��10-3mol•L-1��c��Mg2+��=6��10-4mol•L-1��Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mgCaO����֮�൱�����ʣ���7.1mgMgO����1Lˮ�и��������ʵ���=1.2��10-3mol���൱��CaO����=1.2��10-3mol��56g/mol=67.2mg��1Lˮ��þ�������ʵ���=6��10-4mol���൱������þ����6��10-4mol��40g/mol=24mg������ˮ��Ӳ��=$\frac{67.2mg}{10mg}$+$\frac{24mg}{7.1mg}$=10�㣻
�ʴ�Ϊ��10�㣻
��4��10m3������Ȼˮˮ�к��и��������ʵ���=10��103L��1.2��10-3mol•L-1=12mol��þ�������ʵ���=10��103L��6��10-4mol•L-1=6mol��̼������������ʵ���=10��103L��8��10-4mol•L-1=8mol�������������Ʒ����ķ�ӦΪ��
Mg2++2OH-=Mg��OH��2�� HCO3-+OH-=CO32-+H2O
1 2 1 1 1
6mol 12mol 8mol 8mol 8mol
�������������ʵ���20mol��
��ҪCa��OH��2���ʵ���10mol��������������Ϊ74g/mol��10mol=740g��
ˮ��Һ��Ca2+���ʵ���Ϊ12mol+10mol=22mol��
������Ҫ����� ̼�������Ϊ22mol-8mol=14mol����Ҫ̼���Ƶ�����Ϊ14mol��106g/mol=1484g��
�ʴ�Ϊ��740��1484��
��5���ٵ��ص������������ӷŵ磬���������������ӷŵ磬��ĤA�������ӽ���Ĥ����ĤC�������ӽ���Ĥ���ʴ�Ϊ������
�ڵ缫AΪ�������缫BΪ�����������������ӷŵ��������������������ӣ����������������ӷŵ����������������ӣ���ĤA�������ӽ���Ĥ����ĤC�������ӽ���Ĥ������A�������ԣ�B�������ԣ�C���Լ��ԣ�����pH��С˳��Ϊ��pHa��pHb��pHc��
�ʴ�Ϊ��pHa��pHb��pHc��
���� ���⿼��������ˮ���Ӧ�á���ˮ����������ԭ����ˮ��Ӳ�ȼ���ȣ���Ҫ���������Ϣ��ȷ���㣬��Ŀ�Ѷ��еȣ������ڿ���ѧ���ķ��������ͼ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH2=CHCH2Cl | B�� | CH3OH | C�� | Cl-C2H4-Cl | D�� | Br-CH=CH-Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ӧ�Ƿ��ȷ�Ӧ | B�� | ������Ӧ�����ȷ�Ӧ | ||
| C�� | a+b��c | D�� | a+b��c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
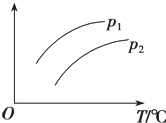 ���ݻ�һ�����ܱ������з������淴Ӧ��A��g��+2B��g��?2C��g����H��0��ƽ���ƶ���ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
���ݻ�һ�����ܱ������з������淴Ӧ��A��g��+2B��g��?2C��g����H��0��ƽ���ƶ���ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | p1��p2��������ָA��������� | |
| B�� | p1��p2��������ָC���������� | |
| C�� | p1��p2��������ָA��ת���� | |
| D�� | p1��p2��������ָ��������ƽ��Ħ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH��5.6��7.0֮��Ľ�ˮͨ����Ϊ���� | |
| B�� | ��ˮ��Դ�����ð���ˮ��Դ�����úͻ�ѧ��Դ�����ã����߿����ۺϽ��� | |
| C�� | ��ҵ���õ�ⱥ���Ȼ�����Һ�ķ����Ƶý����� | |
| D�� | ���ϳ�ϴ�Ӽ����ڱ�ϸ���ֽ⣬�ʲ��ᵼ��ˮ����Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ijʵ����ȤС�����������ʵ��װ�ã�a��bΪֱ����Դ��������ʵ��ʱ�����Ͽ�K1���պ�K2�������������ݲ����������й�������ȷ���ǣ�������
ijʵ����ȤС�����������ʵ��װ�ã�a��bΪֱ����Դ��������ʵ��ʱ�����Ͽ�K1���պ�K2�������������ݲ����������й�������ȷ���ǣ�������| A�� | �Ͽ�K1�պ�K2ʱ���ܷ�Ӧ�����ӷ���ʽΪ��2H++2Cl-$\stackrel{���}{��}$ H2��+Cl2�� | |
| B�� | �Ͽ�K1�պ�K2ʱ�������ء�a��Fe���������Һ��C��b����·������ | |
| C�� | �Ͽ�K1�պ�K2ʱ�����缫������ҺpH������ | |
| D�� | �Ͽ�K2�պ�K1ʱ��Fe�缫���������˷���Ϊ�������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϴ��ʽ�ζ���ʱӦ�ӵζ����Ͽڼ���3��5mL��Ҫʢװ������Һ����б��ת���ζ��ܣ�ʹҺ����ʪ���ڱڣ��ٴ��Ͽڵ������ظ�2��3�� | |
| B�� | �ö��Ե缫���MgCl2��Һ�����ӷ���ʽΪ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH- | |
| C�� | Ϊ��֤���ŵ����ã��������ձ��зֱ�ʢ���������������Һ��ϡ���Ը��������Һ���ֱ����ʯī���õ����������������Ȼ����װ��K2SO4������Һ����֬��U�����˲��������ձ��У�������ƫת��һ��ʱ�����������Һ��ɫ | |
| D�� | �϶������������ú��ڽ���ڶ�����ҹ����ǰ�������˻��������쳣������䲡���Ϊ�³�����������ֱ��С��10�ĸ������³���ɢ�ڿ������γɵķ�ɢϵ���ڽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ȵμ�BaCl2��Һ�ٵμ�HCl��Һ�����ɰ�ɫ������ԭ��Һ����SO42- | |
| B�� | �μ���ˮ��CCl4�������ã��ϲ���Һ����ɫ��ԭ��Һ����I- | |
| C�� | �ýྻ��˿պȡ��Һ������ɫ��Ӧ������ʻ�ɫ��ԭ��Һ����Na+ | |
| D�� | �μ�NaOH��Һ����ʪ���ɫʯ����ֽ�����Թܿڣ���ֽ��������ԭ��Һ����NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg��Al�ڿ����������ȶ�����������к�ǿ�Ŀ���ʴ�� | |
| B�� | ��������ȵIJ�ͬ���أ�һ�����ڲ�ͬ��Ԫ�� | |
| C�� | ��ʯ�ҡ����ۡ��轺��ʳƷ��װ�г��õĸ���� | |
| D�� | ���г��ּ�������Ҫ�ǻ�ѧ��ʴ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com