
����Ŀ�����ݻ�һ�����ܱ������з������淴Ӧ:A(g)+2B(g)![]() 2C(g)����H>0,�����������仯,ֻ���¶ȱ仯ʱ,ij�����¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��������˵����,��ȷ��(����)
2C(g)����H>0,�����������仯,ֻ���¶ȱ仯ʱ,ij�����¶ȱ仯�Ĺ�ϵ��ͼ��ʾ��������˵����,��ȷ��(����)

A. ��p1>p2,�������ʾA����������
B. ��p1<p2,�������ʾC����������
C. ��p1>p2,�������ʾ��������ƽ��Ħ������
D. ��p1<p2,�������ʾA��ת����
���𰸡�C
��������A������ѹǿ��ƽ��������Ӧ������У�A�������������ͣ�����Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ���A�������������ͣ���A����B������ѹǿ��ƽ��������Ӧ������У�C��������������B����C������ѹǿ��ƽ��������Ӧ������У����ʵ�����С����ֶ������壬�����������䣬���ƽ��Ħ����������C��ȷ��D������ѹǿ��ƽ��������Ӧ������У�A��ת��������D����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���ݻ�Ϊ10L�̶����ܱ������г���4molNH3��5molO2�������·�Ӧ��4NH3(g)+5O2(g) ![]() 4NO(g)+6H2O(g)��5s�ﵽƽ�Ⲣ����1molNOʱ��
4NO(g)+6H2O(g)��5s�ﵽƽ�Ⲣ����1molNOʱ��
��1��O2��ת����Ϊ__________________��
��2��������˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����____________��������ĸѡ�
A���������������������� B��c(O2)����
C��5v(O2)�� �� 4v(NO)�� D����ϵѹǿ����
��3�������������¶���Ӧ����______�����������ͨ��Ar����Ӧ����_______��ѡ���������С���������䡱����
��4������Ӧ�ھ����ܱ�ϵͳ�н���ʱ�������������䣬��Ӧ��������������С����ԭ����________��������ĸѡ���
A����Ӧ��ϵ�¶������ߺͣ����Է�Ӧ��������������С
B����Ӧ��Ũ�������ߺͣ����Է�Ӧ������������С
C���÷�ӦΪ���ȷ�Ӧ���ڷ�Ӧ��ʼ�Σ���Ҫ����ϵ�¶����ߵ�Ӱ�죬��Ӧ���������ڷ�Ӧ��Σ���Ҫ��Ũ�ȼ�С���ص�Ӱ�죬��Ӧ���ʼ���
��5����֪���ڿ��滯ѧ��ӦmA + nB ![]() pC + qD��һ���¶��´ﵽ��ѧƽ��ʱ����ƽ�ⳣ��K�ı���ʽΪ��
pC + qD��һ���¶��´ﵽ��ѧƽ��ʱ����ƽ�ⳣ��K�ı���ʽΪ��![]() (���У�CΪ�����ʵ�ƽ��Ũ��)������¶��£�������Ӧ��ƽ�ⳣ��K = ___________________________�������ݱ���г�ʽ�Ӽ��ɣ���
(���У�CΪ�����ʵ�ƽ��Ũ��)������¶��£�������Ӧ��ƽ�ⳣ��K = ___________________________�������ݱ���г�ʽ�Ӽ��ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и����������÷�Һ©��ֱ�ӷ�����ǣ� ��
A. ���������� B. ������Ȼ�̼ C. ������Һ����ɳ D. ���ͺ��Ȼ���ˮ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NAΪ�����ӵ�������ֵ������˵����ȷ����( )
A. 18�˵�D216O�к��е�������Ϊ9NA
B. ��״���£�22.4LSO3���е�ԭ����Ϊ4NA
C. 80ml10mol/L����������MnO2���ȷ�Ӧ��������Cl2������Ϊ0.2NA
D. ����ͭ�����У�������������6.4gʱ����·��ת�Ƶ���Ϊ0.2 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������أ������й�˵���������
A. ��Һ��ʯ��������ȼ�Ϳɼ��ٴ�����Ⱦ
B. �ý��ݹ����������Һ�Ĺ�������ˮ�����������ϩ���Դﵽ���ʵ�Ŀ��
C. ����������������Ҫ�������ʣ�ѪҺ�������Ǻ������ͣ���ʹ�˻�����Ѫ����֢
D. SO2���������ԣ�������Ư��ֽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С����̽��SO2�Ļ�ѧ���ʣ����������ʵ�鷽����
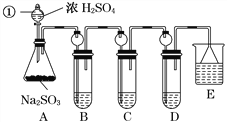
(1)ָ�������������ƣ�______________��
(2)���Aװ�õ������Եķ�����______________________________________________��
(3)װ��B����SO2�������ԣ���B����ʢ�Լ�����Ϊ________��
(4)װ��C��ʢװ��ˮ���Լ���SO2��________�ԣ���C�з�Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
(5)װ��D��ʢװ����Ư��Ũ��Һ��ͨ��SO2һ��ʱ���D�г����˴�����ɫ������ͬѧ�Ƕ�ɫ�����ɷ�������ּ��裺
������һ���ð�ɫ����ΪCaSO3��
��������ð�ɫ����Ϊ__________________________________________________��
���������ð�ɫ����Ϊ�����������ʵĻ���
�����ڼ���һ��ͬѧ�Ƕ�ɫ�����ɷֽ�����̽����������·�����
��ѡ���������Լ�������װ�á��Թܡ��ιܡ������ܵĵ�����������ˮ��0.5 mol��L��1HCl��0.5 mol��L��1H2SO4��0.5 mol��L��1BaCl2��1 mol��L��1NaOH��Ʒ����Һ��
��1������D�г������ˡ�ϴ�Ӹɾ������á�
��ش�ϴ�ӳ����ķ�����____________________________________________________��
��2��������һֻ�ɾ��Թ�ȡ����������Ʒ������________(�Լ�)�����ϴ����ܵĵ������������ܵ���һ�˲���ʢ��________(�Լ�)���Թ��С�������__________________���������һ������
�����������������д�����ɸð�ɫ�����Ļ�ѧ����ʽ��_________________________________��
(6)װ��E��ʢ�ŵ��Լ���________��������__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������л�Ϊͬϵ���һ���� ��������
A. C![]() H
H![]() ��C
��C![]() H
H![]()
B. CH![]() =CH-CH
=CH-CH![]() ��CH
��CH![]() =CH-CH
=CH-CH![]() -CH
-CH![]()
C. ��ϩ�����ϩ
D. ![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Կ��淴Ӧ:A(g)+B(s)![]() C(s)+D(g)����H>0����ͼ��ʾΪ�����淴Ӧ����(v)��ʱ��(t)��ϵ��ʾ��ͼ,�����t1ʱ�̸ı���������:������A;���������;����ѹ;������;������C,����ͼʾ��������(����)
C(s)+D(g)����H>0����ͼ��ʾΪ�����淴Ӧ����(v)��ʱ��(t)��ϵ��ʾ��ͼ,�����t1ʱ�̸ı���������:������A;���������;����ѹ;������;������C,����ͼʾ��������(����)
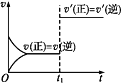
A. �ڢ� B. �٢� C. �ۢ� D. �ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ����������ݼ�¼����ȷ����(����)
A. ��������ƽ����ʱ����NaOH������������ڵ�ֽ�ϣ��Ƶ�����Ϊ10.2 g
B. ��25 mL��ʽ�ζ�����ȡ���������Һ�����Ϊ16.30 mL
C. �ø���Ĺ㷺pH��ֽ��ϡ�����pH��5.1
D. ��10 mL��Ͳ��ȡNaCl��Һ�����Ϊ6.5 mL
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com