
| A��c��K+����c��NO3������c��Cl������c��Ag+����c��I���� |
| B��c��K+����c��NO3������c��Ag+����c��Cl������c��I���� |
| C��c��NO3������c��K+����c��Ag+����c��Cl������c��I���� |
| D��c��K+����c��NO3������c��Ag+����c��Cl������c��I���� |


 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c��CN-����c��Na+�� |
| B��c��CN-����c��HCN�� |
| C��c��HCN��+c��CN-��=0.2mol?L-1 |
| D��c��CN-��+c��OH-��=0.1mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ȼ��� | B��̼����þ | C���������� | D��̼���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cl-��Br-��CrO42- | B��CrO42-��Br-��Cl- | C��Br-��Cl-��CrO42- | D��Br-��CrO42-��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
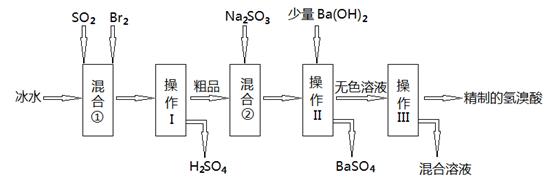
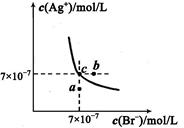
| A������Cl-��Br- �Ļ��Һ�еμ���������Һ��һ���Ȳ���AgBr�ij��� |
| B����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b�� |
| C��ͼ��a���Ӧ����AgBr�IJ�������Һ |
D����t��ʱ��AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)ƽ�ⳣ������816 AgBr(s)+Cl-(aq)ƽ�ⳣ������816 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
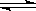 2RAn���л��㣩 + nH2SO4 (ˮ��)
2RAn���л��㣩 + nH2SO4 (ˮ��) | pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| CaSO4 | Mg2(OH)2CO3 | CaCO3 | BaSO4 | BaCO3 |
| 2.6��10-2 | 2.5��10-4 | 7.8��10-4 | 2.4��10-4 | 1.7��10-3 |
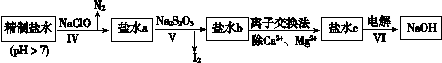
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CaCO3�ܹ��ܽ���CO2��ˮ��Һ�� |
| B��Mg(OH)2���������ᣬ������NH4Cl��Һ |
| C��AgCl�����ڰ�ˮ |
| D��MgSO4��Һ�еμ�Ba(OH)2�õ����ֳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com