
| m |
| n |
| 15g |
| 0.25mol |
| V1 |
| V2 |
| n1 |
| n2 |
| m1 |
| M1 |
| m2 |
| ��M2 |
| 1 |
| 2 |
| 8 |
| M2 |
| 1 |
| 1 |
| V1 |
| V2 |
| n1 |
| n2 |
| m1 |
| M1 |
| m2 |
| M2 |
| 1 |
| 16 |
| 1 |
| M2 |
| 15 |
| 8 |
| P1 |
| P2 |
| n1 |
| n2 |
| m1 |
| M1 |
| m2 |
| M2 |
| a |
| M1 |
| a |
| 16 |
| 4 |
| 11 |
| ��1 |
| ��2 |
| m1 |
| V1 |
| m2 |
| V2 |
| n1��M1 |
| n1Vm |
| n2��M2 |
| n2Vm |
| M1 |
| M2 |
| 16 |
| M2 |
| 1 |
| 4 |
| m |
| V |
| nM |
| nVm |
| M |
| 22.4L/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
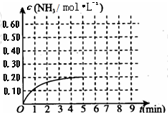 ��2010?��̨һģ��ij�¶�ʱ����һ�ݻ�Ϊ1L���ܱ������У�����0.4mol��N2��1.2mol��H2����һ�������·������·�Ӧ��N2��g��+3H2��g��?2NH3��g����H��0��5minʱ�ﵽƽ�⣬��Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺��1��������ͼ������ӷ�Ӧ��ʼ��ƽ��ʱ��ƽ����Ӧ����v��N2��=
��2010?��̨һģ��ij�¶�ʱ����һ�ݻ�Ϊ1L���ܱ������У�����0.4mol��N2��1.2mol��H2����һ�������·������·�Ӧ��N2��g��+3H2��g��?2NH3��g����H��0��5minʱ�ﵽƽ�⣬��Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺��1��������ͼ������ӷ�Ӧ��ʼ��ƽ��ʱ��ƽ����Ӧ����v��N2��=| c(NH3)2 |
| c(N2)��c(H2)3 |
| c(NH3)2 |
| c(N2)��c(H2)3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ö��Ե缫���2L1.0mol��L-1CuSO4��Һ���ڵ�·��ͨ��0.5mol���Ӻ���������������⣬��·����ͨ����1mo1���ӣ���ʱ��Һ��c(H+)(������Һ�������)Ϊ�� ��
A.1.5mol��L-1 B.0.75mol��L-1
C.0.5mol��L-1 D.0.25mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱���и�����ѧ�ڿ�ѧ���Ի�ѧ�Ծ� ���ͣ�ѡ����
�ö��Ե缫���2L1mol•L��1��CuSO4��Һ���ڵ�·��ͨ��0.5mol���Ӻ�����Դ�������缫����·����ͨ��0.1mol���ӣ���ʱ��Һ�е�c(H��)�ǣ�����ͨ�����Һ��������䣩
A. 0.2mol•L��1 B. 0.6mol•L��1 C. 0.5mol•L��1 D. 0.25mol•L��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com