
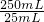 =0.025aVmol������
=0.025aVmol������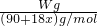 =0.025aVmol�����x=
=0.025aVmol�����x=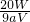 -5��
-5��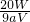 -5��
-5��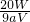 -5��
-5�� ������ᾧ����x��ֵ��
������ᾧ����x��ֵ��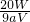 -5���ݴ˽��ѡ�������
-5���ݴ˽��ѡ�������

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�γ���ѧ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
ijͬѧ��18 mol/L��Ũ��������200mL 0.9mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
�� ��1����Ҫ��ȡŨ���� ������ mL��
��2������ƿ��һ�־���ϸ��������ƿ�������侱��ϸ����������������ƿ������Һ��ʱ����Ҫһ�������ĺͼ��ɡ����˽��齫����ƿ��ƿ���Ĵ֣��Ըý������ȷ�����ǣ�������w.w.^w.k.&s.5*u.c.#om��.��.��.Դ.��
| A�������˽���Ľ�������ʹ������ƿ |
| B�����ܰ��˽���Ľ�����Ϊ�ή������ƿ�ľ�ȷ�� |
| C������Ӵ�ƿ�����ɽ�ԭ����������ƿƿ���ϵĿ̶ȸĿ�������ƿ��ƿ���� |
| D�����ؼӴ�ƿ������Ϊ������ƿ��ת��Һ��ʱ��������Һ�嵹��ƿ�⣬�������Һ��Ũ�Ȳ���̫��Ӱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ½������ʯ��ѧ��һ���Ĵ��¿���ѧ�Ծ����������� ���ͣ������
��10�֣�ijͬѧ��18 mol/L��Ũ��������200mL 0.9mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
����1����Ҫ��ȡŨ���� ������ mL(ȷ��С�����һλ)��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ���200mL����ƿ�⣬�������õ��������� ������ �� ������ �ȡ�
��3������ƿ��һ�־���ϸ��������ƿ�������侱��ϸ����������������ƿ������Һ��ʱ����Ҫһ�������ĺͼ��ɡ����˽��齫����ƿ��ƿ���Ĵ֣��Ըý������ȷ������( )
| A�������˽���Ľ�������ʹ������ƿ |
| B�����ܰ��˽���Ľ�����Ϊ�ή������ƿ�ľ�ȷ�� |
| C������Ӵ�ƿ�����ɽ�ԭ����������ƿƿ���ϵĿ̶��߸Ŀ�������ƿ��ƿ���� |
| D�����ؼӴ�ƿ������Ϊ������ƿ��ת��Һ��ʱ��������Һ�嵹��ƿ�⣬�������Һ��Ũ�Ȳ���̫��Ӱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ½���и�һ���Ĵ��¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣�ijͬѧ��18 mol/L��Ũ��������200mL 0.9mol/L��ϡ���ᣬ�������й�ʵ�顣��ش��������⣺
����1����Ҫ��ȡŨ���� ������ mL(ȷ��С�����һλ)��
��2�����Ƹ�ϡ����ʱʹ�õ���������Ͳ���ձ���200mL����ƿ�⣬�������õ��������� ������ �� ������ �ȡ�
��3������ƿ��һ�־���ϸ��������ƿ�������侱��ϸ����������������ƿ������Һ��ʱ����Ҫһ�������ĺͼ��ɡ����˽��齫����ƿ��ƿ���Ĵ֣��Ըý������ȷ������( )
A�������˽���Ľ�������ʹ������ƿ
B�����ܰ��˽���Ľ�����Ϊ�ή������ƿ�ľ�ȷ��
C������Ӵ�ƿ�����ɽ�ԭ����������ƿƿ���ϵĿ̶��߸Ŀ�������ƿ��ƿ����
D�����ؼӴ�ƿ������Ϊ������ƿ��ת��Һ��ʱ��������Һ�嵹��ƿ�⣬�������Һ��Ũ�Ȳ���̫��Ӱ��
��4�������ƹ����У��������ض������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿
������ƿδ���T����������Һ����������ҺŨ�� ���� 0.9 mol��L-1������ڡ��������ڡ���С�ڡ�����ͬ����������ʱ���ӿ̶��ߣ���������ҺŨ�� ���� 0.9 mol��L-1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com