
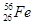 ��2�֣� ��2�����йµ��Ӷԣ�2�֣���
��2�֣� ��2�����йµ��Ӷԣ�2�֣��� ��2�֣���CO��2�֣�
��2�֣���CO��2�֣�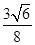 ����0.92�U1����3�֣�
����0.92�U1����3�֣�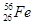 ��
�� ��������CO�γɵľ��嶼�Ƿ��Ӿ��壬���е����ǷǼ��Է��ӣ�CO�Ǽ��Է��ӣ�����CO�ķ��Ӽ�������ǿ�ڵ����ģ���˷е�ϸߵ���CO��
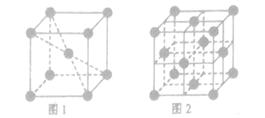
��������CO�γɵľ��嶼�Ƿ��Ӿ��壬���е����ǷǼ��Է��ӣ�CO�Ǽ��Է��ӣ�����CO�ķ��Ӽ�������ǿ�ڵ����ģ���˷е�ϸߵ���CO��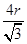 ������ͼ1����ԭ�ӵĿռ���������
������ͼ1����ԭ�ӵĿռ���������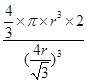 ������ͼ2��֪b2��b2��(4r)2�����b��
������ͼ2��֪b2��b2��(4r)2�����b��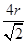 ������ͼ2����ԭ�ӵĿռ���������
������ͼ2����ԭ�ӵĿռ���������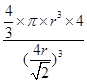 ��������ԭ�ӵĿռ�������֮��Ϊ
��������ԭ�ӵĿռ�������֮��Ϊ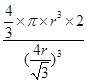 :
: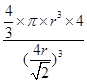 ��
��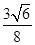 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����X��Y��ɵĻ�����ķе���ܸ�����X��Z��ɵĻ�����ķе� |
| B��X��Z��R����Ԫ���е�����������ϳɵģ�1��1�ͣ������о����й��ۼ� |
| C����ѹ�����£��е㣺X2Z2��XM �������Ӱ뾶��Z��M��R��W |
| D��R 3WM6�������Ϊ��ҵ�������W2Y3��W����ʱ�����ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�� | �����Ϣ |
| X | XԪ���γɵ�һ��ͬ������������Ȼ������Ӳ�����ĵ��� |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬��������ڻ�ҩ |
| Z | Z�Ļ�̬ԭ�Ӻ�����3���ܼ����е��ӣ�����3�������� |
| W | WԪ���γɵ�˫ԭ�ӷ��ӣ�������Ϊ����ɫ���壬һ�ֳ�����ҵԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
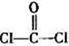 ���������______��
���������______��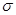 ����____��
����____��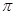 ��������Cԭ�ӵ��ӻ���ʽΪ_______��
��������Cԭ�ӵ��ӻ���ʽΪ_______��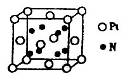 ��ʾ�������Ļ�ѧʽΪ_______��
��ʾ�������Ļ�ѧʽΪ_______���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com