
A��B��X��Y��Z��ԭ���������ε����Ķ�����Ԫ�أ�����A��Yͬ���壬X��Zͬ���壬A��B��A��X�����γ�10�����ӻ����B��Z������������֮��Ϊ2�U3������������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ��졣��ش��������⡣
��1�� X�����ڱ��е�λ����__________________________
��2�� ������Y2X2�ĵ���ʽΪ _________ �������еĻ�ѧ�������� _________
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
��3�� A��X��A��Z�����γ�18�����ӵĻ���������ֻ��������Ӧ����Z�Ļ�ѧ����ʽΪ_____________________________________��
��4�� A�ĵ�����X�ĵ��ʿ��Ƴ����͵Ļ�ѧ��Դ��KOH��Һ���������Һ���������缫���ɶ����̼�Ƴɣ�ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ磬���缫��ӦʽΪ_____________________________________��
��5�� д��������Y2X2��ˮ��Ӧ�����ӷ���ʽ_____________________��
��6�� B�����������ĽṹʽΪ_______________________________��
��1���ڶ����ڢ�A��
��2�� Na2O2 �ĵ���ʽ Na+[:O:O:]2-Na+
�� A��C
��3��H2O2+H2S=S��+2H2O
��4��H2 �C 2e- + 2OH- == 2H2O��2H2 �C 4e- + 4OH- == 4H2O
��5��2Na2O2��2H2O===4 Na����4OH-��O2��
��6�� O=C=O
��������
����������ɳ���������Y2X2��ˮ��Ӧ����X�ĵ��ʣ�����Һ��ʹ��̪��Һ����֪��XΪ����YΪ�ơ�����A��B��A��X�����γ�10�����ӻ������֪AΪ�⡣����X��Zͬ����˵��ZΪ��B��Z������������֮��Ϊ2�U3��֪��B����������Ϊ4������BΪ̼��
���㣺����Ԫ�����ڱ���Ԫ�������ɵ����֪ʶ��


 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��һ5���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ȼ�ϵ�ؿ���ʹ���ں���ɻ��ϣ��䷴Ӧԭ��ʾ��ͼ��ͼ�������й�����ȼ�ϵ�ص�˵������ȷ���ǣ�������
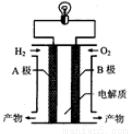
A. A�缫�Ǹ���
B. ���·�е�����B�缫ͨ����������A�缫
C. ����Ϊ����Ⱦ��ˮ�����ڻ����Ѻõ��
D. �õ�ص��ܷ�Ӧ��2H2��O2===2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��ѧ�����п��Ը�һ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪H��H����Ϊ436 kJ��mol-1,N��H����Ϊ391 kJ��mol-1,���ݻ�ѧ����ʽ:N2(g)+3H2(g) 2NH3(g)����H=-92.4 kJ��mol-1,��N��N���ļ�����(����)
2NH3(g)����H=-92.4 kJ��mol-1,��N��N���ļ�����(����)
A.431 kJ��mol-1 B.946 kJ��mol-1
C.649 kJ��mol-1 D.896 kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ���в����������۶�Ӧ��ϵ��ȷ��һ���ǣ� ��
ѡ�� | ���� | ���� | ���� |
A | �������м��뼸��ˮ������ȣ��ټ���Ũ���ᣬѸ�ٽ��� | ������ڣ�������ͣ��γ����ɶ������ | ֻ������Ũ�������ˮ�� |
B | ���ۺ�ϡ�����Ϲ��Ⱥ��ټ���������������ͭ����Һ | ������ɫ���� | ����ˮ������������� |
C | �����������������Һ��ַ�Ӧ��������ϡ�����ữ���ټ�����������Һ | ���ɵ���ɫ���� | �������к�����Ԫ�� |
D | ����ˮ���뱽�в������ | ��ˮ��ɫ | �����巢����ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ��ij�л�����ӵı���ģ�ͣ��йظ����ʵ��ƶϲ���ȷ���ǣ� ��
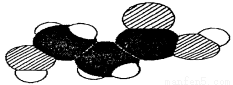
A�������п��ܺ����ǻ� B�������п��ܺ����Ȼ�
C�������п��ܺ��а��� D�������ʵķ���ʽ����ΪC3H6O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��0.4mol������ȫȼ�պ����ɵ�����ȫ������ͨ�ˣ�L 2mol/LNaOH��Һ�У���Na2CO3��NaHCO3�����ʵ���֮��Ϊ
A��3��2 ���� B��2��3 C��2��1 D��1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A���ɷ��ӹ��ɵ�����һ�����й��ۼ�
B���ɷǽ���Ԫ����ɵĻ����ﲻһ���ǹ��ۻ�����
C���Ǽ��Լ�ֻ������˫ԭ�ӵ��ʷ�����
D����ͬԪ����ɵĶ�ԭ�ӷ����еĻ�ѧ��һ�����Ǽ��Լ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ԭ�и߶���ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��ȩ��CH2O�������ᣨC2H4O2���ͱ�ȩ��C3H6O����ɵĻ�����У���Ԫ�ص�����������37%������Ԫ�ص���������Ϊ
A��11% B��28% C��54% D��9%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ԭ�и�һ��ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʽ���һ��ȡ����Ӧ������ֻ�����ֵ��� �� ��
A�� (CH3)2CHCH(CH3)2 B��(CH3CH2)2CHCH3
C�� (CH3)2CHCH2CH2CH3 D��(CH3)3CCH2CH3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com