
| ѡ�õ�����������ĸ�� | ������Լ� |
| A | ϡ���ᡢ����ʯ |
| | |
| | |
| | |
| | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ƷԤ�������õ����в��ἰ�����ε���Һ��
ƷԤ�������õ����в��ἰ�����ε���Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ƿ�еļ������ϩ���ֱ��ȼ���۲�������ɫ���Ƿ��к��� |
| B������ƾ��е�����ˮ����ƾ��м���������ʯ�� |
| C���Ʊ������飨C2H5Cl����������������Ļ�������ڹ��������·�Ӧ |
| D����ȥ�����е���ϩ���壺��ʵ�����Ƶõ���ϩ����ͨ��NaOH ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����Ͳ | B������ƿ | C���ζ��� | D���ձ� |
| ���� | Cu(OH)2 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����pH | 6.0 | 7.5 | 1.4 |
| ������ȫpH | 13 | 14 | 3.7 |
| ʵ�鲽�� | ʵ����� | ʵ�����Ŀ�� |
| ����1 | | |
| ����2 | | |
| ����3 | | |
| ����4 | ϴ�ӳ��� | |
| ����5 | | �õ�FeSO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
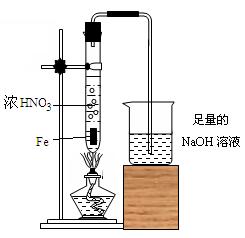
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ����� | ʵ������ | ʵ����� |
| A | ��ij��Һ�м���ϡ���ᣬ��������������ͨ�����ʯ��ˮ�� | ��ɫ��ζ������ʹ����ʯ��ˮ����� | ԭ��Һ�к���CO32? |
| B | ��������ͨ����ˮ�� | ��Һ��ɫ | ����������Ư���� |
| C | ���ۺ�ϡ�����Ϲ��Ⱥ��ټ���������������ͭ����Һ | ������ɫ���� | ����ˮ������������� |
| D | ȡ����ij��ɫ��Һ���ȵμ���ˮ���ټ����������Ȼ�̼�������� | ��Һ�ֲ㣬�²�ʳȺ�ɫ | ԭ��ɫ��Һ��һ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
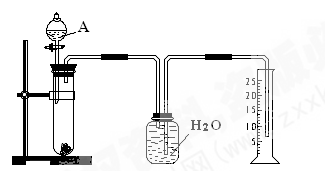
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
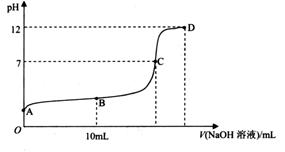
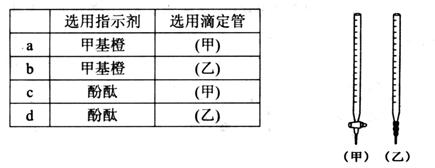
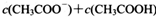 ________
________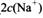 (ѡ�>������<����=��)��
(ѡ�>������<����=��)��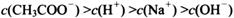
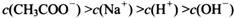
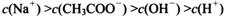
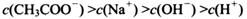
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ۢܢ� | C���٢ڢۢ� | D���٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com