
ijС��������װ�ý����Ҵ���������ʵ�顣��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ ���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�����Ӧ�� ��Ӧ���Ƽ�������ˮԡ���ò���ͬ���������� ���ҵ������� ��
�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л����� ��Ҫ��ȥ�����ʣ����ڻ��Һ�м��� ����д��ĸ����
A���Ȼ�����Һ������B�������� C��̼��������Һ ����D�����Ȼ�̼

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У��Ⱥ��м��Լ����ֺ��зǼ��Լ����ǣ� ��
A������C6H6�� B��CO2 C��Na2O2 D��NH4Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijδ֪��Һ���ܺ�Cl����SO32����Na����SO42����Al3��������Һ���ں�ɫʯ����ֽ�ϣ���ֽ������ȡ������Һ���μ������ữ���Ȼ�����Һ���а�ɫ�������ɣ����ϲ���Һ�еμ���������Һ��������ɫ�����������жϺ������ǣ� ��
A��һ����Cl�� B��һ����SO42�� C��һ��û��Al3�� D��һ��û��SO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1��4-����������һ�ֳ����ܼ���������ͨ�����кϳ�·���Ƶã�����AӦ�ǣ� ��

A.1-��ϩ B.1��3-����ϩ C.��Ȳ D.��ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ƺ�Ԥ������H1N1�ij����в�ҩ����Ч���Գɷ�Ϊ��ԭ�ᣬ�����������ᣬ���й㷺��ɱ������Ч���ṹʽ����ͼ�������й���ԭ���˵������ȷ���ǣ� ��
A������ʽΪC16H18O9
B��1mol��ԭ������ˮ��Ӧ�������4molBr2
C��1mol��ԭ�������5molH2�ӳ�
D��1mol��ԭ�������4molNaOH��Ӧ
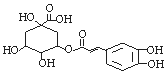
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л������ﻷ���滷���飬����������Ľṹһֱ�ܵ����ۻ�ѧ�ҵ�ע�⣬��ͼ�����Ľṹʾ��ͼ�����й��ڻ����滷�����˵���д������
A�������滷����Ķ���ȡ������4��
B�������滷���鲻�����ǻ������ͬϵ��
C�������滷�����뻷����ϩ��Ϊͬ���칹��
D�������滷�������е�ԭ�Ӿ���ͬһƽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����Ʊ�1��2��������ķ�Ӧԭ�����£�
CH3CH2OH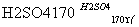 CH2===CH2����H2O
CH2===CH2����H2O
CH2===CH2��Br2�D��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140 ����ˮ�������ѡ�������������������Ҵ��Ʊ�1��2���������װ������ͼ��ʾ��

�й������б����£�
| �Ҵ� | 1��2�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm��3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 |
| 34.6 |
| �۵�/�� | ��130 | 9 | ��116 |
�ش��������⣺
(1)�ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170 �����ң�������ҪĿ����________ ��(����ȷѡ��ǰ����ĸ)
��(����ȷѡ��ǰ����ĸ)
a��������Ӧ b���ӿ췴Ӧ�ٶ�
c����ֹ�Ҵ��ӷ� d�����ٸ�������������
(2 )��
)�� װ��C��Ӧ����________����Ŀ�������շ�Ӧ�п������ɵ��������壻(����ȷѡ��ǰ����ĸ)
װ��C��Ӧ����________����Ŀ�������շ�Ӧ�п������ɵ��������壻(����ȷѡ��ǰ����ĸ)
a��ˮ b��Ũ����
c������������Һ d������̼��������Һ
(3)�жϸ��Ʊ���Ӧ�Ѿ��������������________________________________��
(4)��1��2��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ��________��(��ϡ����¡�)��
(5)��������������δ��Ӧ��Br2�������________ϴ�ӳ�ȥ��(����ȷѡ��ǰ����ĸ)
a��ˮ b������������Һ
c���⻯����Һ d���Ҵ�
(6)�����������������������ѣ�����________�ķ�����ȥ��
(7)��Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ����____________________________��
���ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�Ʊ����������CO2��������ʯ�����ᷴӦ���ɵ������Ⱥ�ͨ��װ���������ʵ���������װ�ã�����װ����Ӧ����װ��(����)
A������NaHCO3��Һ����ˮCaCl2����
B������NaHCO3��Һ��Ũ����
C��Ũ�������NaHCO3��Һ
D������Na2CO3��Һ��ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȩ������ͱ�ȩ��ɵĻ�����У���Ԫ�ص�����������37%����̼Ԫ�ص���������Ϊ�� ��
A��27% B��28% C��54% D������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com