
�������������������γ��������Ҫ���ʡ�ij�������п��ܺ����������ӣ�Na����Ba2����NH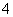 ��Al3����Cl����SO
��Al3����Cl����SO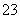 ��SO
��SO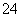 ��NO
��NO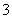 �ȡ�ij�о�С��ȡ�õ�һ���������꣬Ũ�������ó�����Һ�ֳ����ݣ���������ʵ�飺
�ȡ�ij�о�С��ȡ�õ�һ���������꣬Ũ�������ó�����Һ�ֳ����ݣ���������ʵ�飺
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ��Һ����ɫ |
| �ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ�������� |
| ��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ���(V)�����ɵij�������������������ʵ���(n)�Ĺ�ϵ����ͼ |
|
��ش��������⣺
(1)����ʵ�����жϸ������п϶������ڵ�������______________������ȷ����������________________��
(2)д����һ����Һ�μӵ���KI��Һʱ������Ӧ�����ӷ���ʽ��__________________��
(3)��������Һ�μ�NaOH��Һ�����ȣ����������з����˶����Ӧ��д������������Ӧ�����ӷ���ʽ��__________________________________��__________________________��
(4)���ʵ�鷽����������������Ƿ����Cl����___________________________________
______________________________��
(5)��С��Ϊ��̽��NO����������������γɹ��̣�����ƿ�г��뺬������NO��SO2���壬������ͨ��O2��������ѧ��Ӧ����������������ˮ�������������꣬��NO���� ����Ӧ�е�������________________________________________________________��
����Ӧ�е�������________________________________________________________��
������(1)�ɵ�һ����Һ����������֪�������к���NO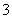 ��������������SO
��������������SO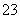 ������NO
������NO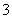 ���棬����Һ��û��SO
���棬����Һ��û��SO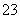 ���ɵڶ�����Һ����������֪�������к���SO
���ɵڶ�����Һ����������֪�������к���SO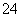 ��û��Ba2�����ɵ�������Һ����������֪�������к���Al3����NH
��û��Ba2�����ɵ�������Һ����������֪�������к���Al3����NH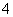 ���ʸ������п϶������ڵ�������SO
���ʸ������п϶������ڵ�������SO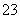 ��Ba2��������ȷ����������Na����Cl����(2)�μӵ���KI��Һʱ��I��������ΪI2��NO
��Ba2��������ȷ����������Na����Cl����(2)�μӵ���KI��Һʱ��I��������ΪI2��NO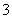 ����ԭΪNO��(3)��������Һ��OH���ֱ���H����Al3����NH
����ԭΪNO��(3)��������Һ��OH���ֱ���H����Al3����NH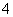 ��Al(OH)3������Ӧ��(4)����Cl��ʱ����Ҫ����������Ba(NO3)2��Һ��SO
��Al(OH)3������Ӧ��(4)����Cl��ʱ����Ҫ����������Ba(NO3)2��Һ��SO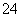 ��ȥ���ų����ţ�Ȼ����HNO3�ữ��AgN
��ȥ���ų����ţ�Ȼ����HNO3�ữ��AgN O3��Һ���м��顣(5)NO�ܱ�������NO2��NO2��ˮ��ת��ΪHNO3��ϡHNO3����SO
O3��Һ���м��顣(5)NO�ܱ�������NO2��NO2��ˮ��ת��ΪHNO3��ϡHNO3����SO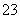 ���յõ��������������꣬���NO�������γɹ�������������
���յõ��������������꣬���NO�������γɹ�������������
�𰸣�(1)SO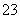 ��Ba2����Na����Cl��
��Ba2����Na����Cl��
(2)6I����2NO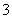 ��8H��===2NO����3I2��4H2O
��8H��===2NO����3I2��4H2O
(3)H����OH��===H2O��NH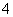 ��OH��
��OH�� NH3����H2O��Al3����3OH��===Al(OH)3����Al(OH)3��OH��===AlO
NH3����H2O��Al3����3OH��===Al(OH)3����Al(OH)3��OH��===AlO ��2H2O(�����)
��2H2O(�����)
(4)ȡ������Һ�μ�������Ba(NO3)2��Һ������ȡ�ϲ����Һ����HNO3�ữ��AgNO3��Һ�����а�ɫ�������ɣ���֤������Cl��
(5)����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�谢���ӵ�����ΪNA��������˵����ȷ����
A��15g��(��CH3)�����еĵ�������9NA
B�����³�ѹ�£�0.1mol�������ȩ�������������̼ԭ����Ϊ0.1NA
C����״���£�1L������ȼ�պ����ɵ���̬����ķ�����Ϊ7/22.4 NA
D�����³�ѹ�£� 1mol�������к���̼̼˫����Ϊ3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ȷ����(����)
A����AlCl3��Һ�еμӰ�ˮ��������ɫ�������ټ���NaHSO4��Һ�������ܽ�
B����Cu(NO3)2��Һ�м���ͭ�ۣ�ͭ�۲��ܽ⣻�ټ���ϡ������Һ��ͭ���ܽ�
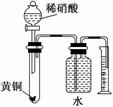
C������ͼ��ʾ��װ�ÿ��Բⶨ��ͭ(Cu��Zn�Ͻ�)��Zn�ĺ���
D����FeCl3��Һ�м���KSCN��Һ����Һ��죬�����������۳������Һ��ɫ��ȥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��FeCl3��Һʴ��ͭ�������·��Ĺ����У���Һ��������Դ���յĹ��̼������£�
�����Һ��Ͷ�������м����ַ�Ӧ�������������Һ��
������Һ�м���һ����ʯ��ˮ��������ҺpH��ͬʱ���������Ŀ�����
��֪��Ksp[Fe(OH)3]��4.0��10��38
�ش��������⣺
(1)FeCl3ʴ��ͭ����Ӧ�����ӷ���ʽΪ________________________________________��
(2)���̢������м����Ҫ������__________________������õ��������Ҫ�ɷ���________���ӹ����з����ͭ����õķ�����___________________________________��
(3)���̢��з�����Ӧ�Ļ�ѧ����ʽΪ________________________________��
(4)���̢��е�����Һ��pHΪ5����������Ũ��Ϊ
________________________________________________________________________
________________________________________________________________________��(��ʽ����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
SiO2��һ�ֻ���ԭ�ϣ������Ʊ�һϵ�����ʡ�����˵����ȷ����(����)

A��ͼ�����з�Ӧ��������������ԭ��Ӧ
B�������εĻ�ѧ�����ȶ���������������ά
C�����������ȥʯӢɰ(��Ҫ�ɷ�ΪSiO2)��������̼���
D����ͨ�������ɴ��ʯ��ʯ��ʯӢɰ�Ƴɵģ����۵�ܸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����±��ṩ��ʵ�������������(�Dz���������ѡ)���ܹ��ﵽ��Ӧʵ��Ŀ�ĵ���(����)
| ѡ�� | ʵ������������� | ʵ��Ŀ�� |
| A | ��ʽ�ζ��ܡ���ʽ�ζ��ܡ��ձ�����ƿ | ��0.01 mol/L��ϡ����궨δ֪NaOH��Һ��Ũ�� |
| B | ��Һ©������ƿ������ܡ����ܡ�����ƿ | �ü�ʯ�Һ�Ũ��ˮ�Ʊ�����NH3 |
| C | ��Ũ�����̼��ϼ��ȣ�ֱ�ӽ����ɵ�����ͨ��������ʯ��ˮ�У�ʯ��ˮ����� | �������������CO2�Ĵ��� |
| D | ��ij��Һ�м���BaCl2��Һ��������ɫ���� | ����SO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�輰�仯����������ִ�������������ף���ش������й����⣺
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
�ٳ�����Ͽˮ���ӡ���ʯӢ���ά�����մ�����������ͨ�������ݹ�̫���ܵ��
A���٢ڢ� B���ۢܢ�
C���ڢۢ� D���٢ۢ�
(3)�����£�SiCl4ΪҺ̬���е�Ϊ57.6�棬�ڿ�����ð�������Ʊ��ߴ��ȹ���м����SiCl4������Һ̬���ʣ���Ҫ�õ��ߴ���S iCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��_______________________________________��
iCl4��Ӧ���õķ�����________���û�ѧ����ʽ����Ҫ���ֽ���SiCl4�ڿ�����ð������ԭ��_______________________________________��
(4)��ҵ�Ͽ���SiCl4(g)�Ʊ����½ṹ�մɵ����裬�䷴Ӧ����ʽΪ
 3SiCl4(g)��2N2(g)��6H2(g)
3SiCl4(g)��2N2(g)��6H2(g)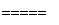 Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
Si3N4(s)��12HCl(g)����H��a kJ/mol(a��0)
�ٸ÷�Ӧ��ƽ�ⳣ������ʽK��______________.
�����ܱպ��������У��ܱ�ʾ������Ӧ�ﵽƽ��״̬����_______ _��
_��
A��3v��(N2)��v��(H2)
B��v��(HCl��4v����4v��(SiCl4)
C����������ܶȱ��ֲ���
D��c(N2)��c(H2)��c(HCl)��1��3��6
����ij�����´ﵽƽ��ʱ��H2��HCl���ʵ���֮��Ϊm��n�����������������䣬�����¶ȴﵽƽ��ʱ��H2��HCl���ʵ���֮��________m��n(�>��������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������X�ķ���ʽΪC5H11Cl����NaOH�Ĵ���Һ����X���ɵ÷���ʽΪC5H10�����ֲ���Y��Z��Y��Z��������ɵõ�2�����顣����������X��NaOH��ˮ��Һ�������������л�����Ľṹ��ʽ������
(����)
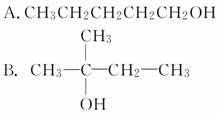
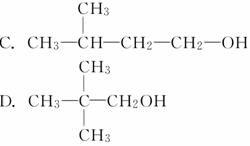
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������H��һ�����ϣ������ڽ����У���������·�ߺϳɣ�

��֪��R��CH===CH2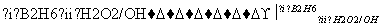 RCH2CH2OH(����B2H6Ϊ������)
RCH2CH2OH(����B2H6������)
��ش��������⣺
(1)11.2 L(��״��)����A�������г��ȼ�տ�������88 g CO2��45 g H2O��A�ķ���ʽ��________________________________________��
(2)B��C��Ϊһ�ȴ��������ǵ�����(ϵͳ����)�ֱ�Ϊ___________��
(3)�ڴ���������1 mol F��2 mol H2��Ӧ������3����1������F�Ľṹ��ʽ��___________________________________��
(4)��Ӧ�ٵķ�Ӧ������_____________________________��
(5)��Ӧ�ڵĻ�ѧ����ʽΪ__________________________��
(6)��G������ͬ�����ŵ�G�ķ�����ͬ���칹�干�����֣��������ֱַ���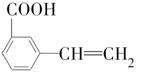 ��
�� ���������ֵĽṹ��ʽ�ֱ���______________
���������ֵĽṹ��ʽ�ֱ���______________
_____________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com