
|
���������ϩ��������Ӧ���Ȼ�ѧ����ʽ(25�棬101 kPa) C2H4(g)��3O2(g)��2CO2(g)��2H2O(l)����H����1411 kJ/mol C2H4(g)��3O2(g)��2CO2(g)��2H2O(g)����H����1329 kJ/mol C2H4(g)��2O2(g)��2CO(g)��2H2O(l)����H����873 kJ/mol C2H4(g)��2O2(g)��2CO(g)��2H2O(g)����H����763 kJ/mol �ɴ��жϣ���ϩ��ȼ������ | |
| [����] | |
A�� |
1411 kJ/mol |
B�� |
1329 kJ/mol |
C�� |
873 kJ/mol |
D�� |
763 kJ/mol |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| A��1411kJ/mol | B��1329kJ/mol | C��873kJ/mol | D��763kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������ʡ���������и߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B���Dz���ˮ��Ӧ�����ʣ���T3��ʱ����A��B�����ʷֱ�����100gˮ�У��պ��Ƴɱ�����Һ��Ȼ���£�A��B���� 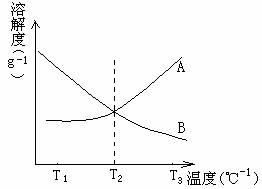 ���ܽ��������ͼ��ʾ�����Ը��������ж�A��B�ıȽ�����ȷ����
���ܽ��������ͼ��ʾ�����Ը��������ж�A��B�ıȽ�����ȷ����
A��T3��ʱ����A�����γɵ���Һ���ʵ���Ũ�����
B������T2��ʱ����A��B���������γɵ���Һ�����ʵ������������
C������T1��ʱ����B�����γɵ���Һ�����ʵ�����������С
D����T3�潵��T1��ʱ��A��B�������γɵ���Һ����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com