
���� ��1����������ǿ�������IJ��ᣬ�ʷ�Ӧ�����ת��ΪHC2O4-����д��C2O42-�Ǵ���ģ�
��2�����Ը�����ؾ���ǿ�����ԣ�������л�ԭ�ԣ�������ǿ��ԭ�Ե����ʣ����Ը�����غͲ��ᷢ��������ԭ��Ӧ���������ӡ�������̼��ˮ��
��3���Ҷ���ֽ�Ļ�ѧ����ʽΪH2C2O4$\frac{\underline{\;\;��\;\;}}{\;}$H2O+CO��+CO2�����ɸ����ʵ����ʿ��Ʋ�װ�õ����ã�
��4����������1��1��Ӧ����NaHC2O4����Һ������˵��HC2O4-�ĵ���̶ȴ�����ˮ��̶ȣ�����NaHC2O4��Һ�����ԣ�˵��HC2O4-�ĵ���̶ȴ�����ˮ��̶ȣ��ɵ�����Ũ�ȼ�Ĵ�С��ϵ��
��� �⣺��1������ǿ�������IJ��ᣬ�ʷ�Ӧ�����ת��ΪHC2O4-��������ǿ��̼�ᣬ
��Ӧ�����ӷ���ʽΪCO32-+H2C2O4=HC2O4-+CO2��+H2O���ʴ�Ϊ��CO32-+H2C2O4=HC2O4-+CO2��+H2O��
��2�����Ը�����ؾ���ǿ�����ԣ�������л�ԭ�ԣ�������ǿ��ԭ�Ե����ʣ����Ը�����غͲ��ᷢ��������ԭ��Ӧ���������ӡ�������̼��ˮ��
���ӷ�Ӧ����ʽΪ��6H++2 MnO4-+5H2C2O4�T10CO2��+2Mn2++8H2O��
�ʴ�Ϊ����ԭ�ԣ�6H++2 MnO4-+5H2C2O4�T10CO2��+2Mn2++8H2O��
��3���Ҷ���ֽ�Ļ�ѧ����ʽΪH2C2O4$\frac{\underline{\;\;��\;\;}}{\;}$H2O+CO��+CO2�����ɸ����ʵ����ʿ��Ʋ�B��CuSO4����������ֽ�����е�ˮ��Cװ�ü������ֽ�����е�CO2��Dװ�õ�Ŀ����Ϊ�˳���CO2����ֹӰ�����ʵ��������жϣ�Eװ�������������壬F��Gװ�������жϲ���ֽ����������CO���ɣ�
�ʴ�Ϊ���ٳ�ȥ��������е�CO2����H2C2O4$\frac{\underline{\;\;��\;\;}}{\;}$H2O+CO��+CO2����
��4����������1��1��Ӧ����NaHC2O4����Һ�����ԣ�˵��HC2O4-�ĵ���̶ȴ�����ˮ��̶ȣ���������1��1��Ӧ����NaHC2O4����Һ������˵��HC2O4-�ĵ���̶ȴ�����ˮ��̶ȣ�����Һ�л�������ˮ�ĵ��룬��H+��C2O42-���������ӵĵ���̶Ƚ�С������HC2O4-��H+������ȷ˳��Ϊc��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
�ʴ�Ϊ����Ӧ����NaHC2O4��HC2O4-�ĵ���̶ȴ�����ˮ��̶ȣ�c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
���� ���⿼���Ѷ���ķֽ��Լ���Һ����Ũ�ȵĴ�С�Ƚϣ���Ŀ��Ϊ�ۺ��Ҿ���һ���Ѷȣ�����ʱע��ץס��Ӧ�������Դ��ƶ����ʿ��ܾ��е����ʣ�


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����л�ѧ���ƻ��ˣ�һ�������˻�ѧ�仯 | |
| B�� | ˮ�ķе������ߣ�����Ϊˮ�еĻ�ѧ���������еĻ�ѧ��ǿ | |
| C�� | ��������Ԫ�ص����Ӱ뾶��С | |
| D�� | ���ۻ�������һ�����ڻ�ѧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��ͼa��ʾװ�ø���NH3���� | |
| B�� | ��ͼb��ʾװ������NaCl��Һ��NaCl���� | |
| C�� | ��ͼc��ʾװ�÷����л�����ˮ�㣬ˮ��ӷ�Һ©���¿ڷų� | |
| D�� | ��ͼd��ʾװ�ò������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCl��CH3COONa��Һ��c��Na+����c��CH3COOH��=c��Cl-�� | |
| B�� | CO2��NH4HCO3��Һ��c��NH4+���Tc��HCO3-��+2c��CO32-�� | |
| C�� | SO2��NaOH��Һ��c��Na+���Tc��SO32-��+c��HSO3-��+c��H2SO3�� | |
| D�� | Cl2��NaOH��Һ��2c��Cl-���Tc��Na+��+c��HClO�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��3a+0.5b��mol | B�� | ��3a+0.5b��mol | C�� | ��3a+0.5b+3p��mol | D�� | ��3a+0.5b-3p��mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʯ��ʯī��Ϊͬ�������� | |
| B�� | CH3-CH2OH�� CH3-O-CH3��Ϊͬ���칹�� | |
| C�� | ${\;}_{2}^{3}$He��${\;}_{2}^{4}$He��Ϊͬλ�� | |
| D�� | ����ʽΪC4H10������Ϊ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
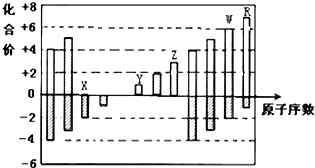
| A�� | ԭ�Ӱ뾶��Z��Y��X | |
| B�� | ��̬�⻯����ȶ��ԣ�R��W | |
| C�� | Y��Z��������������Ӧ��ˮ���������Ӧ | |
| D�� | WX3��ˮ��Ӧ�γɵĻ����������ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
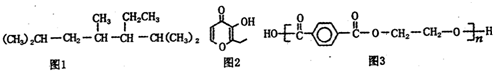
| A�� | ��ϵͳ������������ͼ1�������������2��4��6һ����-5-�һ����� | |
| B�� | ͼ2�л����һ�ַ�����ͬ���칹���ܷ���������Ӧ | |
| C�� | ͼ2�л�����ʹ���Ը��������Һ��ɫ | |
| D�� | ͼ3Ϊ�{���ӻ�����䵥��Ϊ�Ա���������Ҵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{a}{A}$��A-N��mol | B�� | $\frac{a}{A+2m}$��A-N+m��mol | C�� | $\frac{a}{A+2m}$��A-N��mol | D�� | $\frac{a}{A+m}$��A-N+m��mol |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com