







 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g)��H2O(g)�� ��H����49.0 kJ��mol��1
CH3OH(g)��H2O(g)�� ��H����49.0 kJ��mol��1
| A�������¶� | B������He(g)ʹ��ϵѹǿ���� |
| C����H2O(g)����ϵ�з��� | D���ٳ���1 mol CO2��3 mol H2 |
| ���� | �� | �� |
| ��Ӧ��Ͷ���� | 1 mol CO2��3 mol H2 | a mol CO2��b mol H2��c mol CH3OH(g)��c mol H2O(g) |
 ��Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��
��Ҫʹƽ������������ͬ��ֵ����������ȣ�����ʼʱά�ַ�Ӧ������У���c��ȡֵ��ΧΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2(g)��Br2(g)=2HBr(g) ��H����2Q kJ��mol��1 |
| B��H2(g)��Br2(l)=2HBr(g)�� ��H����Q kJ��mol��1 |
C�� H2(g)�� H2(g)�� Br2(g)=HBr(g)��H���� Br2(g)=HBr(g)��H���� kJ��mol��1 kJ��mol��1 |
D��HBr(g)= H2(g)�� H2(g)�� Br2(g)��H���� Br2(g)��H���� kJ��mol��1 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

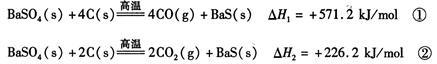
 2CO(g)�ġ�H = kJ/mol
2CO(g)�ġ�H = kJ/mol = ��[��֪��
= ��[��֪���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH4(g)+2H2O(g) ��H��0
CH4(g)+2H2O(g) ��H��0| A������I��II������Ӧ������ͬ |
| B������I��II��CH4�����ʵ���������ͬ |
| C������I��CO2�����ʵ���������II�еĶ� |
| D������I��CO2��ת����������II��CH4��ת����֮��С��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����260.4 kJ��mol��1 | B����260.4 kJ��mol��1 |
| C����611.5 kJ��mol��1 | D����520.8 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH ( g ) ��H��-90.8 kJ��mol��1 ��һ�ݻ��ɱ���ܱ������г���10 mol CO ��20 molH2��CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ20 L��
CH3OH ( g ) ��H��-90.8 kJ��mol��1 ��һ�ݻ��ɱ���ܱ������г���10 mol CO ��20 molH2��CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ20 L��
 CH3OH(g)�ڻ�ѧƽ��״̬ʱ��������ȷ���� ������ĸ����
CH3OH(g)�ڻ�ѧƽ��״̬ʱ��������ȷ���� ������ĸ���� CH3OH(g)��������ͬ�����ʵ��� Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���� ��
CH3OH(g)��������ͬ�����ʵ��� Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���� ��
 CO��g��+H2O��g�� ��H=" +" 41.3 kJ��mol��1�������
CO��g��+H2O��g�� ��H=" +" 41.3 kJ��mol��1����д�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com