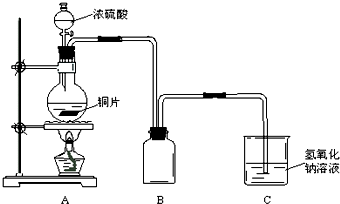
ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飺
��ش��������⣺
��1��B�������ռ�ʵ���в��������װ�ã���δ�����ܻ�ȫ���뽫װ��ͼ����������
��2��ʵ��������ȡ6.4gͭƬ��12mL18mol?L
-1Ũ�������Բ����ƿ�й��ȣ�ֱ����Ӧֹͣ���������ƿ�л���ͭƬʣ�࣬��С���е�ͬѧ��Ϊ����һ����������ʣ�࣮
��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2��+2H
2O
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2��+2H
2O
��
ʵ��������m gͭ�μ��˷�Ӧ������
mol���ᱻ
��ԭ
��ԭ
�����������ԭ������ת�Ƶ��ӵ����ʵ���Ϊ
mol��
�������Լ��У���֤����Ӧֹͣ����ƿ��������ʣ�����
D
D
����д��ĸ��ţ���
A����������Һ B���Ȼ�����Һ C������ D��̼������Һ
��Ϊʲô��һ����������ʣ�൫δ��ʹͭƬ��ȫ�ܽ⣿����Ϊ��ԭ����
ϡ�����ͭ��Ӧ
ϡ�����ͭ��Ӧ
��3��Ϊ�˲ⶨʣ����������ʵ���Ũ�ȣ�����ȤС�����������ʵ�鷽����
����һ����װ��A���������建��ͨ���ѳ�������װ�м�ʯ�ҵĸ���ܣ���Ӧֹͣ���ٴγ�������������������յĶ�������
����������װ��A���������建��ͨ���������������ữ�ĸ��������Һ���ټ����������Ȼ�����Һ�����ˡ�ϴ�ӡ�����Ƶó������������Ƕ�������ת��Ϊ���ᱵ������������
����������ͭ��Ũ����ķ�Ӧ��������װ��A�м���������п�ۣ�����ˮ����ò������������ΪV L���ѻ���Ϊ��״������
ʵ���ϣ����Ϸ���һ����������ȡ������˵��ԭ��
����һ
�����������к���ˮ������������е������������SO2������������ƿ�еĶ�����������ȫ�ų���
�����������к���ˮ������������е������������SO2������������ƿ�еĶ�����������ȫ�ų���
��
������
����������һ�����Ǹ��������Һ�����ữ���õ��������Ȼ�����Ӧ��������
����������һ�����Ǹ��������Һ�����ữ���õ��������Ȼ�����Ӧ��������
��
��������д��ʣ����������ʵ���Ũ�ȵļ���ʽ�����跴Ӧ����Һ�������Ϊ12mL��
��

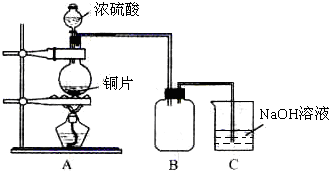
 ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飺
ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飺 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��



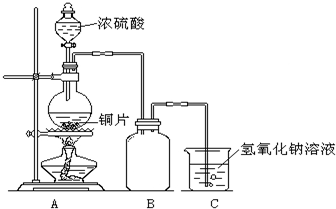
 ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ�������ͼ��ʾװ�ý����й�ʵ�飮��ش�
ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ�������ͼ��ʾװ�ý����й�ʵ�飮��ش� ij��ѧ������ȤС��Ϊ̽�������巢����Ӧ�ķ�Ӧ���Ͳ���ȡ�������屽����������ʵ�飮�����Ҫ��ش�������⣮
ij��ѧ������ȤС��Ϊ̽�������巢����Ӧ�ķ�Ӧ���Ͳ���ȡ�������屽����������ʵ�飮�����Ҫ��ش�������⣮