
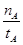 mol��L-1��min-1
mol��L-1��min-1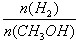 ����
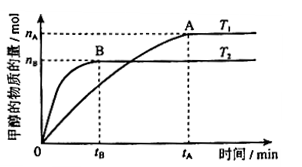
���� CH3OH(g) + H2O(g)����ͼ���֪B�����ȵõ�ƽ�⣬����¶�T2��T1���¶ȸ�ƽ��ʱ�״������ʵ��������ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ��������ڼ״������ɣ�ƽ�ⳣ����С�����ڴ�����ȷ���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״������ʵ���Ϊ
CH3OH(g) + H2O(g)����ͼ���֪B�����ȵõ�ƽ�⣬����¶�T2��T1���¶ȸ�ƽ��ʱ�״������ʵ��������ͣ�˵������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ��������ڼ״������ɣ�ƽ�ⳣ����С�����ڴ�����ȷ���¶�ΪT1ʱ���ӷ�Ӧ��ʼ��ƽ�⣬���ɼ״������ʵ���Ϊ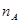 mol����ʱ�״���Ũ��Ϊ
mol����ʱ�״���Ũ��Ϊ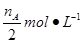 ���������ɼ״���ƽ������Ϊ��v(CH3OH)=
���������ɼ״���ƽ������Ϊ��v(CH3OH)=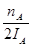 mol��L-1��min-1����ˢٲ���ȷ����Ϊ�¶�T2��T1������A��ķ�Ӧ��ϵ��T1�䵽T2ʱ��ƽ������淴Ӧ�����ƶ���������������Ũ�ȶ�����Ӧ��Ũ�ȣ����Ԣ���ȷ����ѡ)�ۢܡ�
mol��L-1��min-1����ˢٲ���ȷ����Ϊ�¶�T2��T1������A��ķ�Ӧ��ϵ��T1�䵽T2ʱ��ƽ������淴Ӧ�����ƶ���������������Ũ�ȶ�����Ӧ��Ũ�ȣ����Ԣ���ȷ����ѡ)�ۢܡ�

 Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
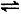 2SO3(g) ?��=��197kJ/mol���ڴ��¶��£���ס��������̶��ݻ����ܱ������зֱ�ͨ��2molSO2��1molO2��1mol SO2��0.5molO2����Ӧ�ﵽƽ��״̬ʱ�ų��������ֱ�ΪQ����Q���������й�ϵ��ȷ����
2SO3(g) ?��=��197kJ/mol���ڴ��¶��£���ס��������̶��ݻ����ܱ������зֱ�ͨ��2molSO2��1molO2��1mol SO2��0.5molO2����Ӧ�ﵽƽ��״̬ʱ�ų��������ֱ�ΪQ����Q���������й�ϵ��ȷ����| A��Q��=0.5Q�� | B��Q��< 0.5Q�� | C��Q��<Q��=197kJ | D��Q��=Q��<197kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3OH��ȼ����Ϊ192.9 kJ��mol��1 |
| B��CH3OH��ȼ����Ϊ133��9 kJ��mol��1 |
| C��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| D�����ݢ���֪��Ӧ��CH3OH(l) ��1/2O2(g) �� CO2(g) ��2H2(g)��DH ����192.9 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
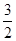 O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1
O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1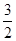 O2��g��=== Fe2O3(s) ��H=" -815.88" kJ? mol-1
O2��g��=== Fe2O3(s) ��H=" -815.88" kJ? mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
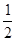 O2(g) == H2O(l) ��H3����285.8kJ��mol-1
O2(g) == H2O(l) ��H3����285.8kJ��mol-1| A����488.3 kJ��mol-1 | B����244.15 kJ��mol-1 | C��+488.3 kJ��mol-1 | D��+244.15 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 Al2O3+Fe ��H��a kJ/mol
Al2O3+Fe ��H��a kJ/mol| A�����ȷ�Ӧ�ڸ����²��ܷ��������a>0 |
| B��̼��ȼ���ȵ���110.5 kJ/mol |
| C��ϡ������ϡNaOH��Һ��Ӧ����1 molˮ���ų�����С��57.3 kJ |
| D����������֪������ת��Ϊ�����Ƿ��ȷ�Ӧ�����Ե������ĺ��������Ȱ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т� | B��ֻ�Т� |
| C��ֻ�Тڢۢ� | D��ֻ�Т٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO2(g) + NaOH(aq) = NaHCO3(aq)��H=" -" (2y-x) kJ��mol��1 |
| B��CO2(g) + NaOH(aq) = NaHCO3(aq)��H=" -" (4x-y) kJ��mol��1 |
| C��CO2(g) + NaOH(aq) = NaHCO3(aq)��H=" -" (2x-y) kJ��mol��1 |
| D��CO2(g) + NaOH(aq) = NaHCO3(aq)��H=" -" (8x-2y) kJ��mol��1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com