
 2Z(g)+W(s)����֪��2molX��1 molY����������У���Ӧ��ij�¶��´ﵽƽ��ʱ��Z�����ʵ���Ϊp mol������˵������ȷ����
2Z(g)+W(s)����֪��2molX��1 molY����������У���Ӧ��ij�¶��´ﵽƽ��ʱ��Z�����ʵ���Ϊp mol������˵������ȷ���� 

 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)���������������������ʼ���ͬ�����������¶��´ﵽƽ�⣬C��ƽ���������е����������Ϊn%����______________________��
A.4 mol A+2 mol B
B.6 mol C+1 mol D
C.1 mol A+0.5 mol B+1.5 mol C+0.5 mol D
D.2 mol A+1 mol B+3 mol C+1 mol D
E.2 mol A+1 mol B+3 mol C+0.5 mol D
(2)��ά�ָ����������䣬���������↑ʼ��ȣ�Ҫ��ﵽƽ��ʱ��C�����ʵ�����ΪW mol����D����ʼ���ʵ���n(D)Ӧ�����������_________________���ú�W�Ĵ���ʽ��ʾ����
(3)�˷�Ӧ��v-tͼ�����ͼ�����������������䣬ֻ���ڷ�Ӧǰ������ʵĴ���������v-tͼ������ͼ�����á���������������գ���a1________________a2����b1________________b2������ͼ����Ӱ�����������_______�ҡ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���ݻ��̶�������ܱ������н��з�Ӧ��2A(g)��B(g) 3C(g)��D(s)����֪��2mol A��1molB����������У���Ӧ��ij�¶��´ﵽƽ��ʱ��C�����ʵ���ΪWmol��C��ƽ���������е��������Ϊn%��
(1)���������������������ʼ���ͬ�����������¶��´ﵽƽ�⣬����һ���ǣ�C�����ʵ���Ϊ2Wmol��C��ƽ���������е����������Ϊn%��������������
A.4molA��2mol B
B.6molC��1mol D
C.1molA��0.5mol B��1.5mol C��0.5mol D
D.2molA��1mol B��3mol C��1mol D
E.2molA��1mol B��3mol C��2mol D
(2)��ά�ָ����������䣬���������↑ʼ��ȣ�Ҫ��ﵽƽ��ʱ��C�����ʵ�����ΪWmol����D����ʼ���ʵ���n(D)Ӧ�������������������������(�ú�W�Ĵ���ʽ��ʾ)��
(3)�˷�Ӧ��v��tͼ�����ͼ�����������������䣬ֻ���ڷ�Ӧǰ������ʵĴ���������v��tͼ������ͼ�����á���������������գ�
��a1������a2����b1������b2������ͼ����Ӱ������������������ҡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ��ɳ�и����������¿���ѧ�Ծ� ���ͣ������
��һ���ݻ��̶�������ܱ������н��з�Ӧ��2A(g)��B(g)  3C(g)��D(s)����֪��2mol A��1mol
B����������У���Ӧ��ij�¶��´ﵽƽ��ʱ��C�����ʵ���ΪWmol��C��ƽ���������е��������Ϊn%��
3C(g)��D(s)����֪��2mol A��1mol
B����������У���Ӧ��ij�¶��´ﵽƽ��ʱ��C�����ʵ���ΪWmol��C��ƽ���������е��������Ϊn%��
(1)���������������������ʼ���ͬ�����������¶��´ﵽƽ�⣬����һ���ǣ�C�����ʵ���Ϊ2Wmol��C��ƽ���������е����������Ϊn%��������������
A.4mol A��2mol B
B.6mol C��1mol D
C.1mol A��0.5mol B��1.5mol C��0.5mol D
D.2mol A��1mol B��3mol C��1mol D
E.2mol A��1mol B��3mol C��2mol D
(2)��ά�ָ����������䣬���������↑ʼ��ȣ�Ҫ��ﵽƽ��ʱ��C�����ʵ�����ΪWmol����D����ʼ���ʵ���n(D)Ӧ�������������������������(�ú�W�Ĵ���ʽ��ʾ)��
(3)�˷�Ӧ��v��tͼ�����ͼ�����������������䣬ֻ���ڷ�Ӧǰ������ʵĴ���������v��tͼ������ͼ�����á���������������գ�
��a1������a2����b1������b2������ͼ����Ӱ������������������ҡ�
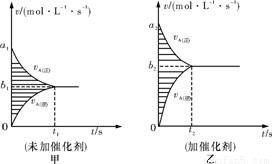
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�߶���ѧ�����п��Ի�ѧ���⣨���� ���ͣ������
��һ���ݻ��̶�������ܱ������н��з�Ӧ��2A(g)+B(g)==3C(g)+D(s)����֪��2molA��1molB����������У���Ӧ��ij�¶��´ﵽƽ��ʱ��C�����ʵ���ΪWmol��C��ƽ���������е��������Ϊn%��
�ſ��϶��������淴Ӧ��һ���������Ѵﵽ��ѧƽ��״̬���� (ѡ�����)��
A����ϵѹǿ���ٱ仯 B��v��(A)��2v��(B)
C����ϵ���ܶȲ��ٱ仯 D����������ƽ����Է����������ٱ仯
�����������������������ʼ���ͬ�����������¶��´ﵽƽ�⣬����һ���ǣ�
C�����ʵ���Ϊ2Wmol��C��ƽ���������е����������Ϊn%����
A��4molA+2molB B��6molC+1molD
C��1molA+0.5molB+1.5molC+0.5molD D��2molA+1molB+3molC+1molD
E��2molA+1molB+3molC+2molD
����ά�ָ����������䣬���������↑ʼ��ȣ�Ҫ��ﵽƽ��ʱ��C�����ʵ�����ΪWmol����D����ʼ���ʵ���n(D)Ӧ����������� ���ú�W�Ĵ���ʽ��ʾ��
�ȴ˷�Ӧ��v��tͼ�����ͼ�����������������䣬ֻ���ڷ�Ӧǰ������ʵĴ�������
��v��tͼ������ͼ�����á�=������������գ���a1 a2����b1 b2������ͼ��
��Ӱ����������� �ҡ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com