
ij����С�����Mg��CO2�ķ�Ӧԭ����̽��Mg��NO2�ķ�Ӧ����������С��ͨ��ʵ��ȷ��Mg����NO2��ȼ�գ����Թ������������ּ��裺
I.����Ϊ���������ΪMgO II.����Ϊ��______________III.����Ϊ��______________
��ش��������⣺������Ϣ��2NO2+2NaOH=NaNO3+NaNO2+H2O
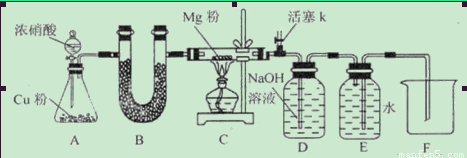
��1����ͼ���Ӻ�������װҩƷǰ��μ���װ�õ�������_____________________________
��2��װ��B��ʢװ�ĸ���������ǣ�����ţ�___________________
��Ũ���� ����ˮCaCl2 �ۼ�ʯ�� ������������
��3����ʼ����k,��A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ����_________________________
��4��E���ռ��������������������ܶ���14,��������__________________
��5��ʵ��õ�����������������ʵ��ǰMg��������1.5���������__________________������C�з����Ļ�ѧ��Ӧ����ʽ��_______________________________________________
��6����ʵ���д�������ȱ�ݣ��Ľ���ʩ��_______________________________________��
��.�������ΪMg3N2 ��.�������ΪMgO��Mg3N2 ��1���رշ�Һ©�������ͻ���K����������ĩ�˲���ˮ�У�����ƿ����C����Ӧ�ܣ��ȣ��������ܿ������ݣ�ֹͣ���ȣ��������н���һ��ˮ������ʾ���������á� ��2���ڢ� ��3���ž�װ���п�������ֹ��������ʵ�顣
��4��N2 ��5������� 4Mg+2NO2 4MgO+N2
3Mg+N2
4MgO+N2
3Mg+N2 Mg3N2
Mg3N2
��6������K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
��������
�����������NO2�к���N��OԪ�أ�����Mg������ȼ�ղ��������MgO��Mg3N2�����ǵĻ��������.�������ΪMg3N2
����.�������ΪMgO��Mg3N2��1����ͼ���Ӻ�������װҩƷǰװ�õ������Լ��鷽���ǣ��رշ�Һ©�������ͻ���K����������ĩ�˲���ˮ�У�����ƿ����C����Ӧ�ܣ��ȣ��������ܿ������ݣ�ֹͣ���ȣ��������н���һ��ˮ������ʾ���������á���2��Cu��Ũ���ᷴӦ����������NO2���������壬����ʹ�ü��Ը����������ų� �ۼ�ʯ�ң�U�ι�װ���ǹ�����������Ũ������Һ�壬����ʹ�ã��ų���Ũ���ᡣ��װ��B��ʢװ�ĸ���������Ǣ���ˮCaCl2 �����������ס���3����ʼ����k,��A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ�����ž�װ���п�������ֹ��������ʵ�顣��4��E���ռ��������������������ܶ���14,��Է�������Ϊ14��2=28����������N2. ��5������ȫת��ΪMgO����Ӧ��Ĺ��������Ƿ�Ӧǰ��(24+16)��24=1.67��.����ȫת��ΪMg3N2����Ӧ��Ĺ��������Ƿ�Ӧǰ��(24��3+14��2)��(24��3)=1.39����ʵ��õ�����������������ʵ��ǰMg��������1.5�������Եõ�����MgO��Mg3N2�Ļ����ʼ�����������C�з����Ļ�ѧ��Ӧ����ʽ��4Mg+2NO2 4MgO+N2
3Mg+N2
4MgO+N2
3Mg+N2 Mg3N2����6����ʵ���д�������ȱ�ݣ�����û��β������װ�á��Ľ���ʩ�ǻ���K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
Mg3N2����6����ʵ���д�������ȱ�ݣ�����û��β������װ�á��Ľ���ʩ�ǻ���K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
���㣺����̽��Mg��NO2�ķ�Ӧ���������ijɷ֡�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�������ɽ��������߿���ѧ����һ�֣� ���ͣ�058
ij����С�����õ�ⷨ���ݵ缫���������ʵ��������ⶨͭ�����ԭ����������ʵ��ԭ��Ϊ���������ͭ��ҺʱCu2+�ڵ缫�Ϸŵ���������������Cu���������������ԭ����������֪�ڲⶨ��������Һ��c(Cu2+)�Ա��ֲ���Ϊ�ˣ��Իش�
(1)ѡ��缫���ϣ�����װ��ʾ��ͼ��
(2)Ϊ��ȷ�ⶨ�缫������ͭ���������������ʵ�鲽����Ⱥ�˳��Ӧ�ǣ�________(ѡ�����в�������ı��)
�ٳ������ǰ�缫�������ڹ��µ���缫�ϵ�ͭ����ϴ��
��������ˮ��ϴ����ĵ缫���ܵ��º�ɵ缫�������
�ݵ��º�ɹ��µ�ͭ����������ٴε��º�ɺ���������أ�
(3)������ǿ��ΪI A��ͨ��ʱ��Ϊt s��ȷ��õ缫��������ͭ������Ϊm g����֪���ӵĵ���Ϊe C�������ӵ�����ΪNA�����г�ͭ�����ԭ�������ļ������ʽ��Mr(Cu)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�(1)�����£�ijˮ��ҺM�д��ڵ������У�Na+��A2-��HA-��H+��OH-�����ڵķ�����H2O��H2A����������ش��������⣺
��д����H2A�ĵ��뷽��ʽ__________________________��
������ҺM��2 mol��L-1NaHA��Һ��2mol��L-1NaOH��Һ�������϶��ã�����ҺM��pH ____7 ���>������<����=��������ҺM�и�����Ũ�ȹ�ϵ��ȷ���� ��
A.c(Na��)��c(A2��)��c(OH��)��c(H��)
B.c(HA��) ��c(H2A) ��c(H��)��c(OH��)
C.c(A2��)��c(HA��)��c(H2A)��1 mol��L-1
D. c(A2��)��c(HA��)��c(OH��)��c(Na��)��c(H��)
(2)����ʱ���������Ƶ��ܶȻ�KSP =4.7��10-6, ����ʱ��9 mL0.02 mol��L��1���Ȼ�����Һ��1 mL pH=13������������Һ��Ϻ�(��Һ�����ֱ�ӼӺ�),��Һ��___ ��������(��С����ޡ�)��
(3) ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ�����ɣ�xCaSO4��yH2O����������ʵ��:��������ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ��
��x:y= ��t2~t3ʱ��ι���Ļ�ѧʽΪ ��t5~t6ʱ��ι������������ԭ���Dz������������壬����һ����ʹƷ����Һ��ɫ�����ʱ����������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ������һѧ���������⿼�Ի�ѧ�Ծ� ���ͣ������
��10�֣�(1)�����£�ijˮ��ҺM�д��ڵ������У�Na+��A2-��HA-��H+��OH-�����ڵķ�����H2O��H2A����������ش��������⣺
��д����H2A�ĵ��뷽��ʽ__________________________��
������ҺM��2 mol��L-1NaHA��Һ��2mol��L-1NaOH��Һ�������϶��ã�����ҺM��pH ____7 ���>������<����=��������ҺM�и�����Ũ�ȹ�ϵ��ȷ���� ��
A.c(Na��)��c(A2��)��c(OH��) ��c(H��)
B. c(HA��) ��c(H2A) ��c(H��)��c(OH��)
C.c(A2��)��c(HA��) ��c(H2A)��1 mol��L-1
D. c(A2��)��c(HA��)��c(OH��)��c(Na��)��c(H��)
(2)����ʱ���������Ƶ��ܶȻ�KSP =4.7��10-6, ����ʱ��9 mL0.02 mol��L��1���Ȼ�����Һ��1 mL pH=13������������Һ��Ϻ�(��Һ�����ֱ�ӼӺ�),��Һ��___ ��������(��С����ޡ�)��
(3) ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ�����ɣ�xCaSO4��yH2O����������ʵ��:��������ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ��
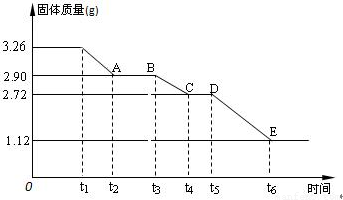
��x:y= ��t2~t3ʱ��ι���Ļ�ѧʽΪ ��t5~t6ʱ��ι������������ԭ���Dz������������壬����һ����ʹƷ����Һ��ɫ�����ʱ����������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�����и�����ѧ���������⿼�Ի�ѧ�Ծ� ���ͣ������
��10�֣�(1)�����£�ijˮ��ҺM�д��ڵ������У�Na+��A2-��HA-��H+��OH-�����ڵķ�����H2O��H2A����������ش��������⣺
��д����H2A�ĵ��뷽��ʽ__________________________��
������ҺM��2 mol��L-1NaHA��Һ��2mol��L-1NaOH��Һ�������϶��ã�����ҺM��pH ____7 ���>������<����=��������ҺM�и�����Ũ�ȹ�ϵ��ȷ���� ��
A.c(Na��)��c(A2��)��c(OH��) ��c(H��)
B. c(HA��) ��c(H2A) ��c(H��)��c(OH��)
C.c(A2��)��c(HA��) ��c(H2A)��1 mol��L-1
D. c(A2��)��c(HA��)��c(OH��)��c(Na��)��c(H��)
(2)����ʱ���������Ƶ��ܶȻ�KSP =4.7��10-6, ����ʱ��9 mL0.02 mol��L��1���Ȼ�����Һ��1 mL pH=13������������Һ��Ϻ�(��Һ�����ֱ�ӼӺ�),��Һ��___ ��������(��С����ޡ�)��
(3) ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ�����ɣ�xCaSO4��yH2O����������ʵ��:��������ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ��
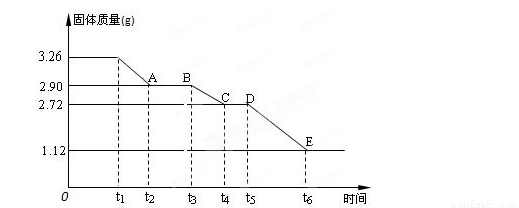
��x:y= ��t2~t3ʱ��ι���Ļ�ѧʽΪ ��t5~t6ʱ��ι������������ԭ���Dz������������壬����һ����ʹƷ����Һ��ɫ�����ʱ����������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��ͬѧ�ķ����ǣ�����Ʒ�ܽ⣬�ӹ����Ȼ�����Һ������ϴ�ӣ�ȡ������ɣ������ù���n g����������̼���Ƶ���������Ϊ_____________����Ca2+��Ba2+����ʹ![]() ������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ����__________________________________��
������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ����__________________________________��
(2)��ͬѧ�ķ�����ͼ��ʾ��
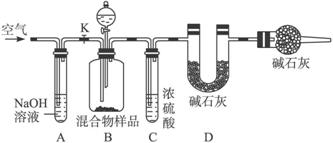
���ݸ�ʵ�鷽������ͬѧ��ʵ���б���ȷ�ⶨ��������_____________�����������Ʒ��ַ�Ӧ��ȫʱ������ͨ�������Ŀ����_____________�����У�װ��A��������_____________��
װ��E��������__________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com