
 2SO3(g)����H=��Q kJ��mol��1��Q��0�������ܱպ�����������ͨ��l mol SO2��0��5 mol O2������һ�ܱպ�����������ͨ��2 mol SO2��1 mol O2����ʼʱ��������������¶Ⱥ�ѹǿ��ͬ����Ӧ�ﵽƽ��ʱ����������������¶Ⱥ�ѹǿ����ͬ����ʱ���зų�������ΪQl kJ�����зų�������ΪQ2 kJ�������й�ϵʽ��ȷ���� ( )
2SO3(g)����H=��Q kJ��mol��1��Q��0�������ܱպ�����������ͨ��l mol SO2��0��5 mol O2������һ�ܱպ�����������ͨ��2 mol SO2��1 mol O2����ʼʱ��������������¶Ⱥ�ѹǿ��ͬ����Ӧ�ﵽƽ��ʱ����������������¶Ⱥ�ѹǿ����ͬ����ʱ���зų�������ΪQl kJ�����зų�������ΪQ2 kJ�������й�ϵʽ��ȷ���� ( )| A��2Q1=Q2<Q | B��2Ql<Q2<Q |
| C��2Q1=Q2=Q | D��Q1<Q2��Q |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
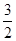 O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1
O2��g��=== Al2O3(s) ��H=" -1" 644.3 kJ? mol-1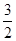 O2��g��=== Fe2O3(s) ��H=" -815.88" kJ? mol-1
O2��g��=== Fe2O3(s) ��H=" -815.88" kJ? mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Т� | B��ֻ�Т� |
| C��ֻ�Тڢۢ� | D��ֻ�Т٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��g����H=" -1367.0" kJ/mol��ȼ���ȣ� |
| B��NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H= +57.3kJ/mol���к��ȣ� |
| C��S��s��+O2��g��=SO2��g����H= -269.8kJ/mol����Ӧ�ȣ� |
| D��2NO2=O2+2NO��H=+116.2kJ/mol����Ӧ�ȣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ӧ��������������������������ʱ���÷�Ӧ�����¼��ɷ��� |
| B������ȼ����Ϊ285.8kJ��mol-1������H2(g)+1/2O2(g)=H2O(g) ��H=��285.8kJ��mol-1 |
| C����1molBa(OH)2��ϡ��Һ��ϡ������ȫ�кͣ��ų�114.6kJ���������Ȼ�ѧ����ʽΪ��OH��(aq)+H��(aq)=H2O(l) ��H =��114.6kJ��mol-1 |
D��2SO2(g)��O2(g)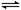 2SO3 (g)����H����QkJ��mol��1(Q��0)����2molSO2(g)������O2����һ�ܱ������У���ַ�Ӧ��ų�������һ��С��QkJ 2SO3 (g)����H����QkJ��mol��1(Q��0)����2molSO2(g)������O2����һ�ܱ������У���ַ�Ӧ��ų�������һ��С��QkJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C + H2O ==CO + H2��H =+131.3kJ��mol��1 |
| B��C(s)+ H2O(g) ==CO(g) + H2(g)��H =+10.94kJ��mol��1 |
| C��C(s)+ H2O(g) ==CO(g) + H2(g)��H = -131.3kJ��mol��1 |
| D��C(s)+ H2O(g) ==CO(g) + H2(g)��H = +131.3kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO2(g) + NaOH(aq) = NaHCO3(aq)��H=" -" (2y-x) kJ��mol��1 |
| B��CO2(g) + NaOH(aq) = NaHCO3(aq)��H=" -" (4x-y) kJ��mol��1 |
| C��CO2(g) + NaOH(aq) = NaHCO3(aq)��H=" -" (2x-y) kJ��mol��1 |
| D��CO2(g) + NaOH(aq) = NaHCO3(aq)��H=" -" (8x-2y) kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
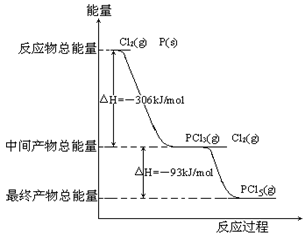
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3OH(l)��3/2O2(g)===CO2(g)��2H2O(l)��H����725.8 kJ��mol��1 |
| B��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)��H����1452 kJ��mol��1 |
| C��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)��H����725.8 kJ��mol��1 |
| D��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l)��H����1452 kJ��mol��1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com