
��20�֣�
��I������ѡ������6�֣�
����˵������ȷ���� ��
A��SO2��O3��PO43����C1O4����Ϊ�ȵ�����
B���������ʧȥһ��H+���γ�CH3������̼ԭ�ӵ��ӻ����ͷ����˸ı�
C��Tiԭ�ӵĺ�������Ų�ʽΪ[Ar]3d34s2
D��CS2��H2O��C2H2����ֱ���ͷ���
E��CH4��BCl3��CO2���Ǻ��м��Լ��ķǼ��Է���
��II����14�֣�
������������һ����Ȼ�粻���ڵ��˹��ϳɳ�Ӳ���ϣ�Ӳ�Ƚ����ڽ��ʯ���dz�Ӳ�������������Ҫ�ɾ�֮һ����ش��������⣺
��1���ڵڶ����ڣ�ԭ�ӵĵ�һ������һ����˵������������� ���Ƚ�����ԭ�ӵĵ�һ�����ܣ�Be B��N O���>����<��������ԭ���� ��
��2����ͼΪ����������ľ���������Ļ�ѧʽΪ ���þ�����Bԭ�����Nԭ�ӵ� ��϶�����þ����ı߳�Ϊa cm����ô�þ�����ܶ�Ϊ g/cm3
��ֻҪ���г���ʽ����
[��Դ:ZXXK]
��3������������ľ���ṹ����ʯ�Ľṹ���ƣ������۵�Ƚ��ʯ�ĵͣ��Է�����ԭ��
��
��4�������������������������ڸ��¸�ѹ���Ʊ��������������ֳơ���ʯī�����ṹ������������ʯī���ƣ�������������Nԭ�ӵ��ӻ��������Ϊ �������Ϸ��ķ����л��������������ƽ��ṹʾ��ͼ���á��𡱴���Nԭ�ӣ��á�����Bԭ�ӣ�ÿ��ԭ�Ӳ�����7������
��20�֣�
��I����6�֣�AE�����1����3�֣�������0�֡���
��II����14�֣�
��1������1�֣���>��1�֣���>��1�֣���Beԭ����2s�������ȫ����״̬��Nԭ����2p������ڰ����״̬���DZȽ��ȶ���״̬�������ǵĵ�һ�����ܸ����������ڵ�ԭ�ӡ���2�֣�
��2��BN��1�֣��������壨1�֣���99.2/(a3NA) ��2�֣�
��3���������������е�����ļ����Ƚ��ʯ������̼̼���ļ���Ҫ�������۵�Ƚ��ʯ�ĵ͡���2�֣�
��4��sp2 ��1�֣�
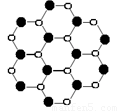 ��2�֣�
��2�֣�
��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
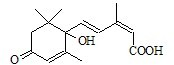 ��I������ѡ����
��I������ѡ����| KMnO4 |
| H+ |
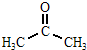 +CH3COOH
+CH3COOH| 120�� |
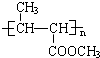
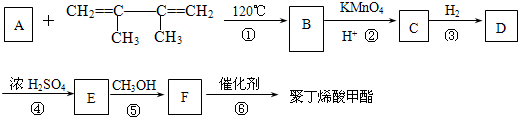




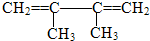 ��ϵͳ����������
��ϵͳ����������



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꺣��ʡ�����и�����ѧ�ڸ߿����в��ԣ�������ѧ�Ծ� ���ͣ������
��20�֣�
��I������ѡ������6�֣�
����˵������ȷ���� ��
A����ϩ��������8���Ҽ���1���м�
B����SiO2�����У�1��Siԭ�Ӻ�2��Oԭ���γ�2�����ۼ�
C��NF3�ķе��NH3�ķе�͵ö࣬����ΪNH3���Ӽ��������NF3ֻ�з��»���
D��NCl3��BC13�����У�����ԭ�Ӷ�����sp3�ӻ�
E���ڡ�����ˮ��ˮ�������������������ı仯�����У����α��ƻ������Ӽ���Ҫ���������������������Ӽ������������Լ�
��II����14�֣�
������ʹ�ý�������ʷ�����У�������ͭ��������֮�����ֽ����㷺Ӧ�õĽ�������ѧ��Ԥ������(Ti)��������Ϊ��δ�����͵Ľ��������Իش��������⣺
��1��TiԪ����Ԫ�����ڱ��е�λ���ǵ�________���ڵ�________�壻���̬ԭ�ӵĵ����Ų�ʽΪ________��
��2����Ti�Ļ������У����Գ��֣�2����3����4���ֻ��ϼۣ������ԣ�4�۵�Ti��Ϊ�ȶ���ƫ���ᱵ�����ȶ��Ժã���糣���ߣ���С�ͱ�ѹ������Ͳ���������ж���Ӧ�ã�ƫ���ᱵ�����о����Ľṹʾ��ͼ��ͼ��ʾ�����Ļ�ѧʽ�� ������Ti4��������λ��Ϊ ��Ba2��������λ��Ϊ ��

��3�������µ�TiCl4���д̼��Գ�ζ����ɫ��Һ�壬�۵�-23.2�棬�е�136.2�棬����TiCl4Ӧ�� ������������ ���塣TiCl4�ڳ�ʪ�������ӷ���ˮ���ð���̣�������Ϊˮ����� ���ɡ�
��4����֪Ti3�����γ���λ��Ϊ6��������ռ乹��Ϊ�������壬����ͼ1��ʾ������ͨ����������ͼ2��ʾ�ķ�������ʾ��ռ乹�ͣ�����A��ʾ���壬M��ʾ����ԭ�ӣ�����λ������[Co(NH3)4Cl2]�Ŀռ乹��ҲΪ�������ͣ����� ��ͬ���칹�壬������ͼ�����н��仭����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ�����и�����ѧ�ڸ߿����У�һ����ѧ���� ���ͣ������
��20�֣�
��I������ѡ���⣨6�֣�
CH3����CH3����CH3��������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ���� ��
A�����Ǿ��ɼ���ȥ��һ����ԭ������
B�����ǻ�Ϊ�ȵ����壬̼ԭ�Ӿ���ȡsp2�ӻ�
C��CH3����NH3��H3O+��Ϊ�ȵ����壬���ι��;�Ϊ������
D��CH3���е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ�����
E������CH3����һ��CH3����CH3����Ͼ��ɵõ�CH3CH3
��II����14�֣�
п��һ����Ҫ�Ľ�����п���仯�������Ź㷺��Ӧ�á�
��1��ָ��п�����ڱ��е�λ�ã� ���ڣ� �壬 ����
��2����������п[CH2OH(CHOH)4COO]2Zn��Ŀǰ�г������еIJ�п����д��Zn2+��̬�����Ų�ʽ �������Ƿ�����̼ԭ���ӻ���ʽ�� ��
��3��Zn2+����NH3�γ�������[Zn(NH3)4]2+����λ��NH3�������� ������Է��ӡ��Ǽ��Է��ӡ�������[Zn(NH3)4]2+�У�Zn2+λ�������������ģ�Nλ����������Ķ��㣬��������ͼ�б�ʾ[Zn(NH3)4]2+��Zn2+��N֮��Ļ�ѧ����


��4������ͼ��ʾп��ij�ǽ���Ԫ��X�γɵĻ����ᄃ��������Zn��Xͨ�����ۼ���ϣ��û�����Ļ�ѧʽΪ ���û�����ľ����۵�ȸɱ��ߵö࣬ԭ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com