
| |||||||||||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
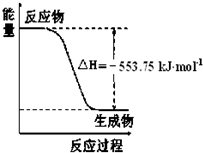 ��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���
��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ����ʵ����ѧ������һ��ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ���ѡ��
��֪��C2H5OH(g)+3O2(g) ="==" 2CO2(g)+3H2O(g) ��H1��-Q1kJ��mol-1��C2H5OH(g) ="==" C2H5OH(l) ��H2��-Q2kJ��mol-1��H2O(g) ="==" H2O(l) ��H3��-Q3kJ��mol-1����ʹ23gҺ��ƾ���ȫȼ�գ��ָ������£���ų�������Ϊ �� ��
| A��Q1��Q2��Q3 |
| B��0.5Q1��0.5Q2��1.5Q3 |
| C��0.5��Q1��Q2��Q3�� |
| D��0.5Q1��1.5Q2��0.5Q3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
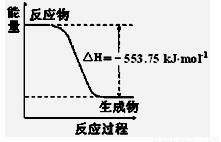
��12�֣���1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵�����ЧӦ��ͬ�������ø�˹���ɻش��������⣺
��֪�� C2H5OH(g)��3O2(g)=2CO2(g)��3H2O(g)����H1����Q1 kJ/mol��C2H5OH(g)=C2H5OH
(l)����H2����Q2 kJ/mol��H2O(g)=H2O(l)����H3����Q3 kJ/mol����ʹ23gҺ̬��ˮ�ƾ���ȫ
ȼ�գ����ָ������£������������зų�������Ϊ ________________ kJ��
��2���������˻����ƻ�潫�й���ͳ�Ļ������˾����Լ��ִ��߿Ƽ���Ϊһ�塣�����
���ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϡ��Իش��������⣺
����ͼ��һ����������ȫȼ������CO2��1mol H2O(l)�����е������仯ͼ����д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ ��
�ڽ������Ѿ����Ƴ�����ȼ�ϵ�أ��õ�صĵ������ҺΪKOH��Һ��д���õ�ظ����ĵ缫��Ӧʽ��__________________________________________��
��ij���������ԭ������Ϊ52.00,��������ص��ý�����һ�ֺ������ε�����ˮ��Һʱ������ÿ�ų� 3360ml����״���������壬������������ 10.4 g���ڸú��������н����Ļ��ϼ�Ϊ ____ ���ڸ�ʵ���У�����������������ʧ����������ı�������������� __ g�������ȷ��0.01 g����?
�ܶ����ѣ�CH3OCH3����һ������ȼ�ϣ�Ӧ��ǰ��������1mol��������ȫȼ������CO2��Һ̬ˮ�ų�1455kJ��������1mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1645kJ���������������У�����Ͷ����ѵ����ʵ���֮��Ϊ ____ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com