

| ||
| ||
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
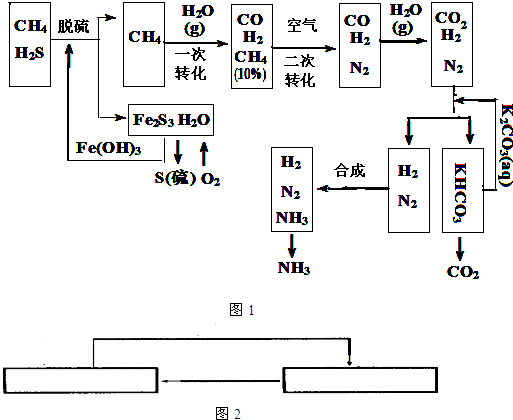
��10�֣�������Ȼ���ϳɰ��Ĺ�������ʾ��ͼ���£�
�����������̣����������գ�
��1����Ȼ������ʱ�Ļ�ѧ����ʽ�� ��
��2��ͼ��CH4�ĵ�һ��ת�������еĻ�ѧ����ʽ�� ��
��3����������������ѭ����һ��K2CO3(aq)ѭ��������N2��H2ѭ�������� ���ѧʽ��ѭ����
��4��K2CO3��aq����CO2��Ӧ�ڼ�ѹ�½��У���ѹ������������ ����ѡ�۷֣���
a.����ԭ�� b.��������ԭ�� c.����к�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��2 2.1���ĺϳ���ϰ���������棩 ���ͣ������
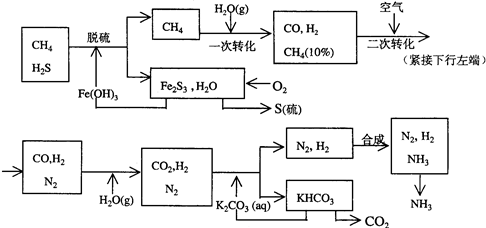
������Ȼ���ϳɰ��Ĺ�������ʾ��ͼ���£�
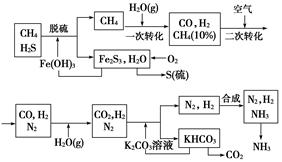
�����������̣����������գ�
(1)n mol CH4��һ��ת�������CO 0.9n mol������H2________mol(�ú�n�Ĵ���ʽ��ʾ)��
(2)K2CO3��Һ��CO2��Ӧ�ڼ�ѹ�����½��У���ѹ������������________(����ĸ����)��
a����������ԭ����b��ƽ���ƶ�ԭ����c������к�ԭ��
(3)��KHCO3�ֽ�õ���CO2��������________(д��CO2��һ����Ҫ��;)��
(4)��������������ѭ����һ��Fe(OH)3ѭ��������K2CO3��Һѭ��������________ѭ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com