
 ������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״��������A�Ľṹ��ʽΪ
������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״��������A�Ľṹ��ʽΪ ������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��
������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��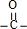 ���ݴ���д���
���ݴ���д��� ������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��
������Է���������ȣ���A�����ụΪͬ���칹�壬A������Na2CO3��Ӧ�ų�CO2�����Ȼ�-COOH��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2����״�����������ǻ�-OH���������ǻ�-OH��ĿΪ2���������Ľṹ��֪���к���C=O˫����A�л�����-CHO��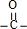 ������������A�ĽṹΪ��HOCH2CH��OH��CHO��CO��CH2OH��2��
������������A�ĽṹΪ��HOCH2CH��OH��CHO��CO��CH2OH��2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ�Ӻ˴Ź����ף�PMR)���о��л�������ṹ����Ҫ����֮һ�������о��Ļ���������У�����λ����ȫ��ͬ����ԭ�ӣ�����Hԭ��)�ں˴Ź������г���ͬһ���źŷ壬���з��ǿ�������Hԭ�ӵ���Ŀ�����ȡ�����ȩ��CH3CHO)�ں˴Ź���������2���źŷ壬��ǿ��֮��Ϊ3��1��
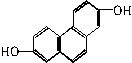
��1���ṹ��ʽ��ͼ��ʾ���л����PMR���й۲쵽���ַ��ǿ��֮��Ϊ___________��
��2��ʵ���п��Ը���PMR���й۲쵽����ԭ�Ӹ����ķ�ֵ�����ȷ���л���Ľṹ���绯ѧʽΪC3H6O2����״�л�������ں˴Ź������ϸ����ķ���ȶ�ǿ�Ƚ������֣����е����ַ�ֵǿ��֮��Ϊ��3��3����2��2��1��1�����ƶϳ����Ӧ�Ľṹ��ʽ��
��__________________________________��
��__________________________________��
��3������ú˴Ź����ķ������о�C2H6O�Ľṹ�����Ҫ˵�����ݺ˴Ź����Ľ����ȷ��C2H6O���ӽṹ�ķ���_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�챱���и߶�12���¿���ѧ�Ծ� ���ͣ������
��28�֣���ϩ����Ҫ�Ļ���ԭ�ϣ����Ʊ��ܶ��л��ʵ������ȡ��ϩ��װ������ͼ��ʾ��
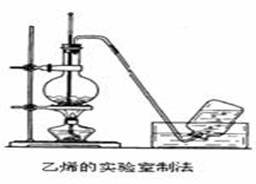
��1����ʵ������ȡ��ϩ�Ļ�ѧ��Ӧ����ʽΪ_______________________________________���ڴ˷�Ӧ����______________��Ӧ����ƿ�м������Ƭ��������______________________
��2��ij��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ��������ͼʵ��װ�ã���ȷ�����������������C2H4��SO2���ش��������⣺
�١�I��II����IVװ�ÿ�ʢ�Ų�ͬ���Լ������У�

I�� IV��_____�����Լ�����ţ�
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
�ڡ���˵��SO2������ڵ�������____________________________________
�ۡ�ȷ��������ϩ��������_____________________________________
(3) ʵ���п��Ը���ԭ�Ӻ˴Ź����ף�PMR���й۲쵽����ԭ�Ӹ����ķ������ȷ���л���
�Ľṹ���ú˴Ź����ķ������о�C2H6O�Ľṹ�����������Ϊ___________(���ֵ)����
ΪCH3CH2OH �����������Ϊ___________(���ֵ)����ΪCH3OCH3��
��4�����Ҵ�����ȡ������������д�йط�Ӧ����ʽ��ע����Ӧ��������
�����Ҵ�����������ȩ��_____________________________________________________
����ȩ������Cu��OH��2��Ӧ��_________________________________________________
�������ữ������ᣩ
���������Ҵ�������������_________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(7��)ԭ�Ӻ˴Ź����ף�PMR�����о��л�������ṹ����Ҫ����֮һ�������о��Ļ���������У�����λ����ȫ��ͬ����ԭ�ӣ�����Hԭ�ӣ��ں˴Ź������г���ͬһ���źŷ壬���з��ǿ�������Hԭ�ӵ���Ŀ�����ȡ��� ��ȩ��CH3CHO���ں˴Ź���������2���źŷ壬��ǿ��֮��Ϊ3:1��
(1)CH3CH2COOH��PMR���й۲쵽���ַ��ǿ��֮��Ϊ ��
(2)ʵ���п��Ը���PMR���й۲쵽����ԭ�Ӹ����ķ�ֵ�����ȷ���л���Ľṹ����֪�л���W(C3H6O3)����NaHCO3��Ӧ���ҷ�ֵǿ��֮��Ϊ3:1:1:1�����ƶϳ����Ӧ�Ľṹ��ʽΪ ��
(3)��֪�л���A��W�����Ժ��ֱ�����ϣ�ֻҪ����һ������ȫȼ�պ������ˮ������Ҳһ����
�ٷ�����������Է���������С���л���A�� (��д�ṹ��ʽ)��
����A���������Է���������ȣ���������Na2CO3��Ӧ�ų�CO2��1mol A���������Ʒ�Ӧ�ɲ���22.4L H2(��״��)����A�Ľṹ��ʽΪ �� ��
(4)W��������;������ȡ��
![]()
W
д����Ӧ�ڷ������������ �� д��C�Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��28�֣���ϩ����Ҫ�Ļ���ԭ�ϣ����Ʊ��ܶ��л��ʵ������ȡ��ϩ��װ������ͼ��ʾ��
��1����ʵ������ȡ��ϩ�Ļ�ѧ��Ӧ����ʽΪ_______________________________________���ڴ˷�Ӧ����______________��Ӧ����ƿ�м������Ƭ��������______________________
��2��ij��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ��������ͼʵ��װ�ã���ȷ�����������������C2H4��SO2���ش��������⣺
�١�I��II����IVװ�ÿ�ʢ�Ų�ͬ���Լ������У�
I�� IV��_____�����Լ�����ţ�
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
�ڡ���˵��SO2������ڵ�������____________________________________
�ۡ�ȷ��������ϩ��������_____________________________________
(3) ʵ���п��Ը���ԭ�Ӻ˴Ź����ף�PMR���й۲쵽����ԭ�Ӹ����ķ������ȷ���л���
�Ľṹ���ú˴Ź����ķ������о�C2H6O�Ľṹ�����������Ϊ___________(���ֵ)����
ΪCH3CH2OH�����������Ϊ___________(���ֵ)����ΪCH3OCH3��
��4�����Ҵ�����ȡ������������д�йط�Ӧ����ʽ��ע����Ӧ��������
�����Ҵ�����������ȩ��_____________________________________________________
����ȩ������Cu��OH��2��Ӧ��_________________________________________________
�������ữ������ᣩ
���������Ҵ�������������_________________________________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com