
���� ��1����ϡ�����ữ���ټ������KI��Һ����������Ӻ͵�����������Һ�з������з�Ӧ���ɵⵥ�ʺ�ˮ����ϵ���غ��ԭ���غ���ƽ��д��
��2����������ˮ������жϣ�Na2S2O3Ϊǿ�������Σ�
��3���ⵥ������������ɫ�����ⵥ�����ģ���ɫ��ȥ��
��4�����ݷ�Ӧ�ҳ���Ӧ��ϵʽ��Ȼ������������ݼ�����ӵ�ʳ����Ʒ�еĵ�Ԫ�غ�����
��5�����ζ������Ӷ�������ȡ�ı���Һ�������
��� �⣺��1�����������£������ӱ�����������������ɵⵥ�ʣ����ӷ�Ӧ����ʽΪ��IO3 -+5I-+6H+�T3I2+3H2O��
�ʴ�Ϊ��IO3 -+5I-+6H+�T3I2+3H2O��
��2��Na2S2O3Ϊǿ�������Σ���Ϊ������������ˮ�⣬Na2S2O3��Һ�������ԣ����Եζ�ʱNa2S2O3��ҺӦ���ڼ��Եζ����У�
�ʴ�Ϊ����ʽ�ζ��ܣ�
��3��ʹ�õ�����Ϊָʾ����������ۣ���Һ����ɫ����Na2S2O3��Һ�ζ���I2��Ӧ��ϣ���Һ��ɫ��ɫ���ζ��յ�����Ϊ����Һ��ɫ��ɫ��������ڲ��ָ���
�ʴ�Ϊ����ɫ��ɫ��Ϊ��ɫ���Ұ���Ӳ��ָ�ԭɫ��
��4��20mL��Һ����Na2S2O3�����ʵ���Ϊ1.00��10-3mol/L��0.02L=2.00��10-5mol��
����IO3-+5I-+6H+=3I2+3H2O��I2+2S2O32-=2I-+S4O62-����
IO3-��3I2��6S4O62-��
1 6
xmol 2.00��10-5mol
����x=$\frac{2.00��1{0}^{-5}mol}{6}$
�ʼӵ�ʳ����Ʒ�еĵ�Ԫ�غ�����$\frac{2.00��1{0}^{-5}mol��127g/mol}{6Wg}$��103mg/kg=$\frac{127}{300W}$mg/kg��
�ʴ�Ϊ��$\frac{127}{300W}$��
��5�����ζ������Ӷ�������ȡ�ı���Һ��������������Ԫ�صĺ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� �����Ե�����е�IJⶨΪ���忼����������ԭ�ζ���Ӧ�á���ʵ��ԭ��������ʵ��װ�õ����ۣ�ע�����յ⼰�仯����������ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�


 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4 | B�� | 5 | C�� | 6 | D�� | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Է�������Ϊ���� | |
| B�� | �������������Է���������ż�� | |
| C�� | �������ĺ������������Է�������Ϊż�� | |
| D�� | �ӡ�ȩ�������ᡢ������Է�������Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
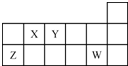
| A�� | ԭ�Ӱ뾶�Ĵ�С˳��rZ��rY��rX | |
| B�� | Y�ļ��⻯��ˮ��Һ������ | |
| C�� | Y���⻯����W���⻯�ﲻ�ܷ�����Ӧ | |
| D�� | X��Y��Z��W����Ԫ�ص�����������Ӧˮ���������ǿ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
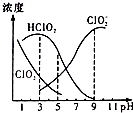 ֯��Ư���������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮25��ʱ������ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л�����������˵��������� ����������������
֯��Ư���������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮25��ʱ������ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л�����������˵��������� ����������������| A�� | 25��ʱ��HClO2�ĵ���ƽ�ⳣ������ֵKa=10-6 | |
| B�� | ʹ�ø�Ư�������pHΪ3.0 | |
| C�� | 25��ʱ����Ũ�ȵ�HClO2��Һ��NaClO2��Һ�������Ϻ����Һ�У�c��HClO2��+2c��H+��=c��ClO2-��+2c��OH-�� | |
| D�� | ���¶���NaClO2��Һ�У�c��Na+����c��ClO2-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʾ�Ҵ�ȼ���ȵ��Ȼ�ѧ����ʽ����H�ľ���ֵ��ȷ����C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��g����H=-1367.0kJ•mol-1 | |
| B�� | NH4Al��SO4��2��Һ�м���Ba��OH��2��ҺʹSO42-��ȫ������Al3++2SO42-+2Ba2++4OH-=AlO2-+2BaSO4��+2H2O | |
| C�� | ��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO4-+6H++5H2O2=2Mn2++5O2��+8H2O | |
| D�� | �������ữ�ij�ɫ���ظ���أ�K2Cr2O7����Һ���Ҵ�������������Ͳ���ɫ���۸����������ڼ���Ƿ�ƺ��ʻ��2Cr2O72-+3C2H5OH+16H+��4Cr3++3CH3COOH+11H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
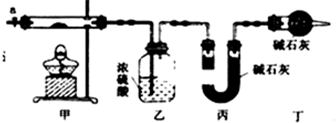
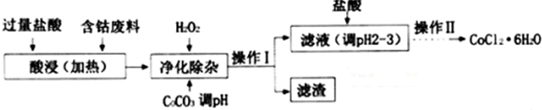
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 |
| ��ʼ������pH | 2.3 | 7.5 | 7.6 | 3.4 |
| ��ȫ������pH | 4.1 | 9.7 | 9.2 | 5.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2OCH3 | B�� | CH3CH��OH��CH3 | C�� | CH3CH2 CH2CH2OH | D�� | CH3CH2CHO |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com