
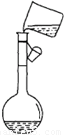
 �ж϶�������ҺŨ�ȵ�Ӱ�죮
�ж϶�������ҺŨ�ȵ�Ӱ�죮

 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
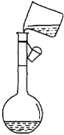 ijͬѧ��NaCl��������100mL 2mol/L��NaCl��Һ����ش��������⣮
ijͬѧ��NaCl��������100mL 2mol/L��NaCl��Һ����ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijͬѧ��NaCl��������100mL 2mol/L��NaCl��Һ����ش��������⣮
ijͬѧ��NaCl��������100mL 2mol/L��NaCl��Һ����ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ���Ȼ��ƹ�������0.4mol/L��NaCl��Һ500mL���ش��������⣺
��1����������Ϊ���ձ���������ƽ��ҩ�ס���ͷ�ιܣ�����Ҫ��Щ��������������ɸ�ʵ�飬��д������ ���� ��
��2����ɸ�ʵ�鲽�� �ټ��� �ڳ���NaCl g �� ��ת��
��ϴ�Ӳ�ת�� ���� ��ҡ��
��3����������������Ƶ�CuSO4��ҺŨ���к�Ӱ�죿���á�ƫ��ƫС������Ӱ�족��д��
A������ƿ������ϴ�Ӻ������������ˮ
B�����ù����ձ���������δϴ��
C������ʱ���ӿ̶���
D����ҡ����Һ����Һ����ڿ̶��ߣ��ٲ���ˮ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com