
Ϊȷ��ij��Һ��������ɣ���������ʵ�飺
�ٲⶨ��Һ��pH����Һ��ǿ���ԡ�
��ȡ������Һ����ϡ���ᣬ�����̼�����ζ����ʹ����ʯ��ˮ����ǵ����塣
���ڢ�������Һ���ٵμ�Ba(NO3)2��Һ��������ɫ������
��ȡ��������Һ�ϲ���Һ�����μ�Ba(NO3)2��Һ������ʱ���ٵμ�AgNO3��Һ��������ɫ������
��������ʵ�飬�����Ʋ���ȷ���ǣ� ��
A.һ����![]() B.����ȷ��
B.����ȷ��![]() �Ƿ����
�Ƿ����
C.����ȷ��Cl-�Ƿ�� D.����ȷ��![]() �Ƿ����
�Ƿ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| (c+d) | 2 |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ��ʼ������pH | ������ȫ��pH | |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Zn2+ | 5.9 | 8.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ��Һ�п϶�����Fe2+��Mg2+��SiO32- | B�����������ɫ������ܺ���CO2��ԭ��Һ�п��ܺ���CO32- | C��ԭ��Һ�п϶�����K+��Fe2+��NO3-��SO42-��I- | D��Ϊȷ���Ƿ���Cl-����ȡԭ��Һ����������������ϡ���ᣬ�۲��Ƿ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������ѧ�ڵڶ��ν��Բ��Ի�ѧ�Ծ� ���ͣ������
��10�֣�A��H����ͼ��ʾ��ת����ϵ������A���Σ�B��C��D��E��F�ڳ��³�ѹ�¾�����̬���ʣ���Ӧ�ܡ��ݾ�����Һ�н��У�ת���з�Ӧ������ȥ��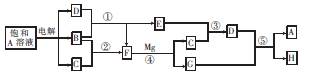
�Իش��������⣺
��1������A�Ļ�ѧʽΪ �����ʱ������ӦʽΪ .
��2����Ӧ�۵Ļ�ѧ����ʽΪ ��
��3����Ӧ�ݵ����ӷ���ʽΪ ��
��4��þ�������γɶ��ֺϽ�Ϊ��ȷ��ij�Ͻ���Ʒ�ijɷ֣�С��ͬѧ�����ͼ��ʾ��ʵ�鲽 �裬ȷ���Ͻ���ɡ�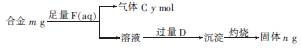
����ȷ���Ͻ���ɵ��������� ������ĸ����
a��m��n b��m��y c��n��y
�����Ͻ���Mg�����ʵ�������Ϊx�����������ʵ���Ϊ7 mol������ͼ������y��x�仯�����ߡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com