
ͼ1�ǵ�A����һ��ǿ�ȴ̼�������F�������̵�ʾ��ͼ��ͼ2Ϊͼ1��D�ṹ�ķŴ�ʾ��ͼ����ش�?
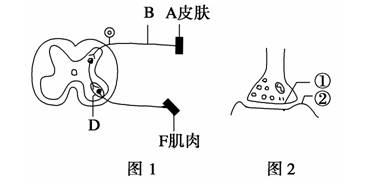
��1��ͼ2�Ľṹ������ ���ṹ�ڵ������� ��?
��2������άB��A�е�ϸС��֦���� �������Aʱ������F����������Ϊ ����������ij�˵Ľŵף�����ʹ���IJ�λ�� �����˻�δ�о�����ʹ֮ǰ������̧�����ȷ�Ӧ�����ڸ÷�Ӧ��������λ��____________��
��3�����˷�������άB�ϴ���ʱ���˷ܲ�λ��Ĥ��������ĵ�λ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
| �� |
| �� |
| һ������ |
 +2��n-1��H2O
+2��n-1��H2O| һ������ |
 +2��n-1��H2O
+2��n-1��H2O



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
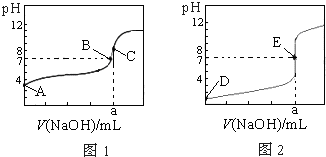 ��ͼΪ��������0.1000mol?L-1NaOH��Һ�ζ�20.00mL0.1000mol?L-1�����20.00mL 0.1000mol?L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵����ȷ���ǣ�������
��ͼΪ��������0.1000mol?L-1NaOH��Һ�ζ�20.00mL0.1000mol?L-1�����20.00mL 0.1000mol?L-1��������ߣ�����HA��ʾ�ᣬ�����жϺ�˵����ȷ���ǣ�������| A��ͼ1�ǵζ���������� | B��B��E״̬ʱ������Һ������Ũ�Ⱦ�Ϊc��Na+��=c��A-�� | C��C��E״̬ʱ����Ӧ���ĵ���n��CH3COOH��=n��HCl�� | D����0 mL��V��NaOH����20.00 mLʱ����Ӧ��Һ�и�����Ũ�ȴ�С˳��һ����Ϊc��A-����c��Na+����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
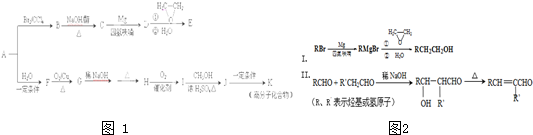
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������д��һ�и������ϣ��ڶ����¿���ѧ�Ծ��������棩 ���ͣ������
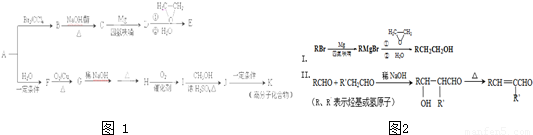
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com