������A������ͬ�����£�����ı仯��ֱ��Ӱ���ܶȣ�
B�������������������ʵ���Ũ�Ȼ��㷽����������жϣ�
C����90% H
2SO
4��Һ��10% H
2SO
4��Һ�������ϣ���Һ�ܶȴ���1������������һ������50%��
D��ʢ�е�����Ȼ�����Թܵ�����ˮ����ʱ��ˮ�����������ƿ������������ˮ��Ӧ����ʽΪ��3NO
2+2H
2O=2HNO
3+NO�����ݷ���ʽ֪��ˮ�������ƿ��
������C=
������Һ�����ʵ���Ũ�ȣ�
���
�⣺A��ijҺ�����ʵ�Ħ����������18 g?mol
-1������ͬ�����£�����ı仯��ֱ��Ӱ���ܶȣ��������ܶȲ�һ����ˮ�����Ҵ�Ħ������Ϊ46g/mol�����ܶ�С��1����A����
B��c mol?L
-1 KCl��Һ���ܶ�Ϊ��g/cm
3�����KCl��Һ����������=
��100%����B��ȷ��
C����90% H
2SO
4��Һ��10% H
2SO
4��Һ��������ϣ��������������غ㣬����������һ������50%�������ܶȴ���1����90% H
2SO
4��Һ��10% H
2SO
4��Һ�������ϣ�����������һ������50%����C����
D����ͬ�����£�����������������ʵ�����ȣ��Ȼ��⼫������ˮ����ʢ���Ȼ�����Թܵ�����ˮ����ʱ��ˮ�����������ƿ��
����������ˮ��Ӧ����ʽΪ��3NO
2+2H
2O=2HNO
3+NO�����ݷ���ʽ֪��ˮ�������ƿ��
����Һ�е����������ᣬ�����ʵ����Ƕ���������
��
������ƿ�������1L����n��HCl��=n��NO
2��=
��
��Һ�����ʵ����ʵ����ֱ��ǣ�n��HCl��=
��n��HNO
3��=
��
��
��Һ������ֱ���V��HCl��=1L��V��HNO
3��=
��
����C=
֪����Һ�����ʵ���Ũ����ȣ�������Ũ��֮��Ϊ1��1����D����
��ѡB��




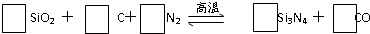
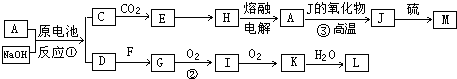 A��J���ճ������г��������ֽ����������ֽ�����NaOH���ԭ��أ�A��������F�����������嵥�ʣ������������µ�ת����ϵ�����ֲ��P������ȥ����
A��J���ճ������г��������ֽ����������ֽ�����NaOH���ԭ��أ�A��������F�����������嵥�ʣ������������µ�ת����ϵ�����ֲ��P������ȥ����