
���и���14��Ԫ�صĵ縺�ԣ�
| Ԫ�� | Al | B | Be | C | Cl | F | Li | Mg | N | Na | O | P | S | Si |
| �縺�� | 1.5 | 2.0 | 1.5 | 2.5 | 3.0 | 4.0 | 1.0 | 1.2 | 3.0 | 0.9 | 3.5 | 2.1 | 2.5 | 1.8 |
������Ԫ��������֪ʶ������и��⡣
(1)ͬһ�����У������ң�Ԫ�صĵ縺����________��ͬһ�����У����ϵ��£�Ԫ�صĵ縺����________�����ԣ�Ԫ�صĵ縺����ԭ�������ĵ�����________�仯��
(2)������Ԫ���У��ɵ縺������Ԫ����縺����С��Ԫ���γɵĻ�����Ļ�ѧʽΪ________���õ���ʽ��ʾ�û�������γɹ��̣�____________________________________��
(3)���з��ϡ��Խ��߹���Ԫ����Li��________��Be��________��B��________�����ǵ����ʷֱ���һ���������ԣ���ԭ����____________________��д��Be(OH)2��ǿ�ἰǿ�Ӧ�����ӷ���ʽ��____________________________________��
(4)һ����Ϊ�����ɼ�Ԫ�ؼ�縺�Բ�ֵ����1.7ʱ�γ����Ӽ������ɼ�Ԫ�ؼ�縺�Բ�ֵС��1.7ʱ���γɹ��ۼ����ж�Mg3N2��SO2��AlCl3��CS2��MgO�����ӻ����ﻹ�ǹ��ۻ����
���ӻ�����___________________________________________
���ۻ�����___________________________________________
������(1)�縺��������������ͬԪ�ص�ԭ�ӶԼ��ϵ����������Ĵ�С���縺�ԵĴ�С������Ϊ�ж�Ԫ�ؽ����Ժͷǽ�����ǿ���ij߶ȣ��縺��Խ��ǽ�����Խǿ��ͬһ���ڴ����ҵ縺��������ͬһ������ϵ��µ縺����С��Ԫ�صĵ縺����ԭ�������ĵ������������Ա仯��
(2)�縺������Ԫ��ΪF���縺����С��ΪNa���仯����ΪNaF��
(3)���з��϶Խ��߹����Ԫ��ΪLi��Mg��Be��Al��B��Si���ǵĵ縺����ֵ�������ѧ�������ƣ���Al(OH)3�������ԣ�Be(OH)2Ҳ�������ԡ�
(4)���������Ϣ�жϳ�Mg3N2�縺�Բ�ֵΪ3.0��1.2��1.8>1.7��MgO�縺�Բ�ֵΪ3.5��1.2��2.3>1.7��Mg3N2��MgOΪ���ӻ����SO2�縺�Բ�ֵΪ3.5��2.5��1.0<1.7��AlCl3�縺�Բ�ֵΪ3.0��1.5��1.5<1.7��CS2�縺�Բ�ֵΪ2.5��2.0��0.5<1.7��SO2��AlCl3��CS2Ϊ���ۻ����
�𰸡�(1)����С��������
(2)NaF��
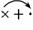
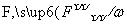 ������Na��[
������Na��[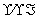

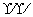 ]��
]��
(3)Mg��Al��Si���縺����ֵ���
Be(OH)2��2H��===Be2����2H2O
Be(OH)2��2OH��===BeO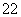 ��2H2O
��2H2O
(4)Mg3N2��MgO
SO2��AlCl3��CS2


 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�����ϩ�;���ϩ����������ȷ����(����)
A�����߶���ʹ��ˮ��ɫ����������
B������Ϊͬ���칹��
C���������ʽ��ͬ
D����ͬ��������������ȼ�պ���������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A��Ԫ�����ڱ���Ԫ�������ɵľ��������ʽ
B��Ԫ�����ڱ����к���һ����
C��Ԫ�����ڱ��У�ijһ��������Ԫ�ص��������ܸ�ijһ��������Ԫ�ص��������
D��Ԫ�����ڱ������������ڸ�������ԭ�ӵĵ��Ӳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ԭ�ӵļ۵��ӹ����У���һ��������С����(����)
A��2s22p4 B��3s23p4
C��4s24p4 D��5s25p4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ӣ�P��S����Mg��Ca����Al��Si����ԭ���У��ֱ��ҳ���һ�����ܽϴ��ԭ�ӣ�����3��ԭ�ӵ�ԭ��������ӣ������(����)
A��40 B��41 C��42 D��48
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У�����������ϩ�ļӳɲ������(����)
A��CH3CH2Br������������ B��CH3CH3
C��CH3CHCl2 D��CH3CH2OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����CH4��CH2===CH2��CH��CH�����������(����)
A���ֱ�ͨ����ˮ
B���ֱ�ͨ�����Ը��������Һ
C���ֱ��ڿ����е�ȼ
D���ֱ�ͨ��ʢ�м�ʯ�ҵĸ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������ҩ�Ϳ�ϵ�����Ӧ�ù㷺��
(1)���й��ڻ�������˵������ȷ����________��
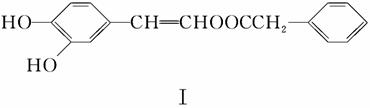
A����FeCl3��Һ��������ɫ
B���ɷ���������Ӧ��������Ӧ
C�������巢��ȡ���ͼӳɷ�Ӧ
D��1 mol��������������2 mol NaOH��Ӧ
(2)��Ӧ����һ����ϩ��ֱ���Ʊ������������·�����
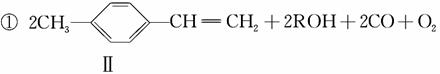

�������ķ���ʽΪ________��1mol�����������________mol H2ǡ����ȫ��Ӧ���ɱ���������
(3)���������ɷ����廯��������ֱ�ͨ����ȥ��Ӧ��ã���ֻ�Т�����Na��Ӧ����H2����Ľṹ��ʽΪ________(д1��)���ɢ����ɢ�ķ�Ӧ����Ϊ________��
(4)�ۺ��� �������Ʊ�Ϳ�ϣ��䵥��ṹ��ʽΪ ________���������Ʒ�Ӧ�ٵķ�����������ϩΪ�л���ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ_________________________________________________
�������Ʊ�Ϳ�ϣ��䵥��ṹ��ʽΪ ________���������Ʒ�Ӧ�ٵķ�����������ϩΪ�л���ԭ�Ϻϳɸõ��壬�漰�ķ�Ӧ����ʽΪ_________________________________________________
_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧУ����С�����һ��һС���Թܺ�����������Һ���ס�������ͬѧ�������Ʒ�����Ƥ�����������һ��װ������ͼ����ȡ���ռ�һ�Թ�������
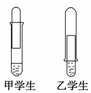
(1)�ĸ�ѧ����Ƶ�װ�ñȽϺ�����____(��ס����ҡ�)����һ���װ�ò�������ԭ
��________________________________________________________________________
________________________________________________________________________��
(2)�ñȽϺ�����װ����ȡ������Ҫʹ���������ռ��������Թܣ�Ӧ�ò�ȡ�Ĵ�ʩ��
________________________________________________________________________��
(3)����������Щ������Ʒ���Լ����������Ƥ������������Һ���ʵ�飿
________________________________________________________________________
________________________________________________________________________��
(4)�ⶨH2�Ϳ����������ı�ը��Χʵ������������ȡ10֧���Թܣ�����ʢˮ90%(�������)��80%����������ˮ�������ռ�H2������ֱ���Թܿ��ƽ��ƾ��ƻ��棬ʵ�������£�
| H2�������/% | 90 | 80 | 70 | 60��20 | 10 | 5 |
| �����������/% | 10 | 20 | 30 | 40��80 | 90 | 95 |
| ��ȼ���� | ���� ȼ�� | ���� ȼ�� | ����ը | ǿ��ը | ����ը | ��ȼ�� ����ը |
������ʵ�������ۣ��������ſ������ռ�H2�������Թܵ����ƽ����棬���ֻ�������ġ�����������ʾ�ռ���H2�Ѵ�����˵�����������壺________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com