
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO ��SO
��SO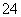 ��Cl����I����HCO
��Cl����I����HCO ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ��� | ��Һ���ɫ |
| ��ȡ��������Һ����Ũ������ͭƬ��Ũ������� | ����ɫ����������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ����BaCl2��Һ | �а�ɫ�������� |
| ��ȡ�����ϲ���Һ����AgNO3��Һ | �а�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ����NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
�ɴ��жϣ�
(1)��Һ�п϶����ڵ�������________����Һ�п϶������ڵ�������________��
(2)Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ�鼰��Ӧ���������ӵ�����(˵��ʹ���Լ������ƣ�����д��ϸ��������)��________________________________________________��
�����������ɢ�֪��Һ�����ԣ�����HCO ���ɢ�֪��NO���ɣ�ԭ��Һ�к�NO
���ɢ�֪��NO���ɣ�ԭ��Һ�к�NO ����һ������Fe2����I��(���л�ԭ��)���ɢ�֪��SO
����һ������Fe2����I��(���л�ԭ��)���ɢ�֪��SO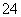 ���ڣ���ԭ��Һ����Ba2�����ɢܲ���ȷ���Ƿ�Cl��(�������Cl��)���ɢ�֪��Mg2����Al3����
���ڣ���ԭ��Һ����Ba2�����ɢܲ���ȷ���Ƿ�Cl��(�������Cl��)���ɢ�֪��Mg2����Al3����
���𰸡���(1)Mg2����Al3����NO ��SO
��SO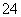 ��Fe2����Ba2����I����HCO
��Fe2����Ba2����I����HCO
(2)����ɫ��Ӧȷ���Ƿ�K������ԭ��Һ��Ba(NO3)2��Һ��ϡ�����AgNO3��Һȷ���Ƿ�Cl��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�õؿ���ij��ҪԪ�������Ķ��ֲ�Ʒ���ִ��߿Ƽ���ռ����Ҫλ�ã������ѧ���ִ�������������Ҫ���á����磺
(1)�����оƬ����Ҫ�ɷ���__________________��
(2)���ά����Ҫ�ɷ���______________________��
(3)ĿǰӦ������̫���ܵ�صĹ��ת��������__________________��
(4)����������������������������������������ɸ�����һ���Ϊ______ �Ļ���
��5��һЩ��ɱȽϸ��ӵĹ����Σ��������������ʽ��ʾ����ʾ˳��Ϊ�����ý�������
��ϻ��ý���������������衢ˮ����(NaAlSi3O8)��дΪ��������ʽΪ________________________��
��6������Ĺ����β�Ʒ���մɡ�������ˮ�ࡣ����������Ҫ�����ǽ������ϡ���ͨ����������_________��________________��__________________Ϊ����ԭ���Ƴɹ����β�Ʒ����дԭ�ϵ����ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л��������зе���ߵ���
A�����顡�������������� B����ϩ
C���Ҵ� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У�����ȡ����Ӧ����
��CH3CH===CH2��Br2�D��CH3CHBrCH2Br
��2CH3CH2OH��O2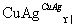 2CH3CHO��2H2O
2CH3CHO��2H2O
��CH3COOH��CH3CH2OH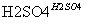
CH3COOCH2CH3��H2O
��C6H6��HNO3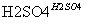 C6H5NO2��H2O
C6H5NO2��H2O
A���٢� B���ۢ�
C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʲ�����һ���Լ�ͨ����ѧ��Ӧ���ֵ���(����)
A��MnO2��������CuO��������FeO
B��(NH4)2SO4 K2SO4 NH4Cl
C��AgNO3 KNO3 Na2SO3
D��Na2CO3 NaHCO3 K2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ����ij�л����ܽ���������NaOH��Һ�У������̪����Һ�ʺ�ɫ�����5 min����Һ��ɫ��dz���ټ������������ԣ���������ɫ���塣ȡ��������ŵ�FeCl3��Һ�У���Һ����ɫ������л��������(����)
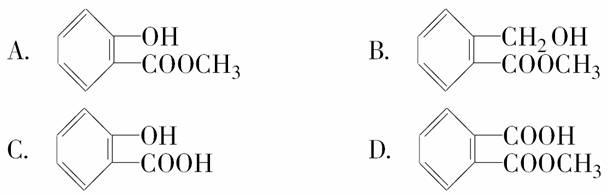
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ᡢ�Ȼ��ơ��������ƺ���ˮ������Һ���������������ǵ�һ���Լ��ǣ� ��
A��AgNO3��Һ B����̪��Һ C����ɫʯ����Һ D������ʳ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��MnO2��Ũ������ȡ�������������������������ͭ�۷�Ӧ��ȡ������
��ˮCuCl2 �� װ������ͼ��ʾ��
װ������ͼ��ʾ��
��ش��������⣺
(1) ʵ��ǰ�������װ���������Եķ�����
(2) B��ѡ�õ��Լ��DZ���ʳ��ˮ����������
C��ѡ�õ��Լ��� ���������� �� (3) E�з�Ӧ�Ļ�ѧ����ʽ�� ��
������������������ˮ���õ� ɫ��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Cl2��SO2��2H2O =H2SO4��2HCl��Ӧ������˵����ȷ���ǣ� ��
��Cl2���������� ��SO2�������� ��Cl2��������
��Cl2������ԭ��Ӧ�� ��SO2���л�ԭ�� ����Cl2����������
A��ֻ�Т٢ڢޡ� B��ֻ�Тڢۢ�
C��ֻ�Тڢܢݢ� D��ֻ�Т٢ڢܢݢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com