
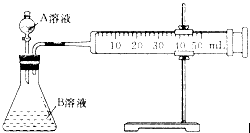

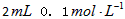 H2C2O4��Һ����ȡ��֧�Թܸ�����
H2C2O4��Һ����ȡ��֧�Թܸ����� KMnO4��Һ������֧�Թֳܷ�����(����һ֧ʢ��H2C2O4��Һ��KMnO4��Һ���Թ�)��һ�������ˮ�У���һ�������ˮ�У�����һ��ʱ��ֱ��ϲ�����¼��Һ��ɫ����ʱ�䡣��ʵ����ͼ̽��__________�Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͬѧʼ��û�п�����Һ��ɫ����ԭ����_____________________________________��
KMnO4��Һ������֧�Թֳܷ�����(����һ֧ʢ��H2C2O4��Һ��KMnO4��Һ���Թ�)��һ�������ˮ�У���һ�������ˮ�У�����һ��ʱ��ֱ��ϲ�����¼��Һ��ɫ����ʱ�䡣��ʵ����ͼ̽��__________�Ի�ѧ��Ӧ���ʵ�Ӱ�죬������ͬѧʼ��û�п�����Һ��ɫ����ԭ����_____________________________________�� 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
 Fe2+��aq��+3OH-��aq��
Fe2+��aq��+3OH-��aq�� Fe2+��aq��+3OH-��aq��
Fe2+��aq��+3OH-��aq��| ���� | FeS | MnS | CuS | PbS | HgS | ZnS |
| Ksp | 6.3��10-18 | 2.5��-13 | 1.3��10-36 | 3.4��10-28 | 6.4��10-53 | 1.6��10-24 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
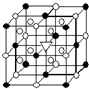 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
| 80m-135n |
| 18n |
| 80m-135n |
| 18n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
�� ����˫ϩ�ϳɷ�Ӧ���ֳ�Ϊ��Diels-Alder��Ӧ�����磺
����˫ϩ�ϳɷ�Ӧ���ֳ�Ϊ��Diels-Alder��Ӧ�����磺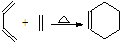 ��
�� ��������A�ĽṹʽΪ��
��������A�ĽṹʽΪ�� ��CH2=CH-COOH
��CH2=CH-COOH ��CH2=CH-COOH
��CH2=CH-COOH

 �У�D���ʿɷ�����Ӧ����һ�������г��ø߷��ӣ��仯ѧ����ʽΪ��
�У�D���ʿɷ�����Ӧ����һ�������г��ø߷��ӣ��仯ѧ����ʽΪ��



 ��
�� ������ɫҺ�壬���������п��������������������ʵ���
������ɫҺ�壬���������п��������������������ʵ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �̷����壨FeSO4?7H2O����ҽҩ������Ѫ����ijͬѧ��KMnO4��Һ�ζ��̷����壨FeSO4?7H2O����Ʒ�����ʲ��� KMnO4��Ӧ��������Ԫ�غ������вⶨ��
�̷����壨FeSO4?7H2O����ҽҩ������Ѫ����ijͬѧ��KMnO4��Һ�ζ��̷����壨FeSO4?7H2O����Ʒ�����ʲ��� KMnO4��Ӧ��������Ԫ�غ������вⶨ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ�����и���3��������⻯ѧ�Ծ��������棩 ���ͣ������
(13��)�������Ļ������ڹ�ҵ�������ճ������ж��й㷺����;��
��1���ڶ������У�������������������Ӧ�ų����������и�ֽ�÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߡ�
��2����֪��2Fe2O3(s)��3C(s)��3CO2(g)��4Fe(s)? ��H��+468.2 kJ��mol-1
C(s)+O2(g)��CO2(g) ��H=-393.5 kJ��mol-1��
��Fe(s)��O2 (g)��Ӧ����Fe2 O3 (s)���Ȼ�ѧ����ʽΪ��_____________________��
��3������KMnO4��Һ�ζ�Fe2+��Ũ�ȣ���Ӧ�����ӷ���ʽ���£�5Fe2����MnO4����8H����5Fe3����Mn2����4H2O
��KMnO4��ҺӦʢ���ڣߣߣߣߣߵζ����У�
���жϴﵽ�ζ��յ�������ǣߣߣߣߣߣ�
���������ữ��0.020 00 mol��L-1��KMnO4��Һ�ζ�ijFeSO4��Һ���յ㣬ʵ�����ݼ�¼���±���
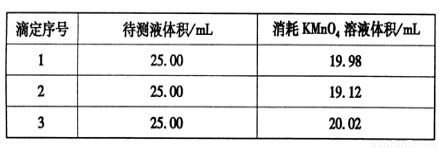
��������ݲ����㣬��FeSO4��Һ�����ʵ���Ũ��Ϊ�ߣߣߣߣߡ�
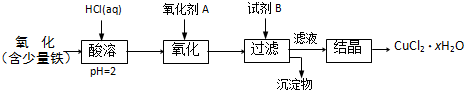
��4���������ײ���ZnFe2Ox�������ڳ�ȥ��ҵ�����е�ijЩ�������ȡ�²��Ϻͳ�ȥ������ת����ϵ����ͼ��
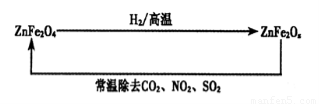
����֪ZnFe2O4��H2��Ӧ�����ʵ���֮��Ϊ2:1����ZnFe2Ox��x=�ߣߣߣߣߣ�
����ZnFe2Ox��ȥSO2�Ĺ����У��������ǣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com