��2010?ʯ��ɽ��һģ������N
2��H
2����ʵ��NH
3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
��1����֪��N
2��g��+O
2��g���T2NO��g����H=+180.5kJ?mol
-1N
2��g��+3H
2��g��?2NH
3��g����H=-92.4kJ?mol
-12H
2��g��+O
2��g���T2H
2O��g����H=-483.6kJ?mol
-1����17g��������������ȫ����һ�����������ˮ�������ų�������Ϊ
226.3kJ
226.3kJ
��
��2��ij����С���о��������������������£��ı���ʼ�����������ʵ�����N
2��g��+3H
2��g��?2NH
3��g����Ӧ��Ӱ�죬ʵ������ͼ2��ʾ����ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�����
��ͼ����T
1��T
2�Ĺ�ϵ�ǣ�T
1��
��
T
2�������������������=������ȷ������
�ڱȽ���a��b��c����������ƽ��״̬�У���Ӧ��N
2��ת������ߵ���
c
c
������ĸ��ţ���
������ʼ��ϵ�м���N
2�����ʵ���Ϊ
molʱ����Ӧ�İٷֺ�������������ݻ�Ϊ1L��n=3mol��Ӧ�ﵽƽ��ʱH
2��ת����Ϊ60%����������£�T
2������Ӧ��ƽ�ⳣ��K=
2.08��mol?L-1��-2
2.08��mol?L-1��-2
��
��3��N
2O
3��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��
��һ���¶��£��ں����ܱ�������N
2O
3�ɷ������з�Ӧ��2N
2O
3?4NO
2��g��+O
2��H��0�±�Ϊ��Ӧ��ij�¶��µIJ���ʵ��������50s��NO
2��ƽ����������Ϊ
0.0592mol?L-1?s-1
0.0592mol?L-1?s-1
| V/s |
0 |
50 |
100 |
| c��N2O3��/mol?L-1 |
5.00 |
3.52 |
2.48 |
������H
2��O
2��������Na
2CO
3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N
2O
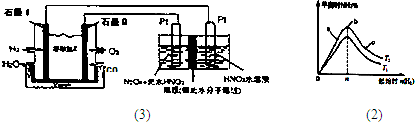
3��װ����ͼ1��ʾ������YΪCO
2��д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ
H2+CO32-�TCO2+H2O+2e-
H2+CO32-�TCO2+H2O+2e-





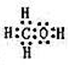
 H++HO2-
H++HO2- H++HO2-
H++HO2-