��2011?�ٴ���ģ�⣩�ִ���п�ķ����ɷ�Ϊ��ʪ�������࣬������������п�ĸ���Ʒ�����ڸ߶��Խ������Իش�����������⣮
��1������п�ǽ���п����Ҫ��ZnS��ͨ����ѡ������ʹ��ת��Ϊ����п���ٰ�����п�ͽ�̿��ϣ��ڹķ�¯�м�����1373-1573K��ʹп�����������Ҫ��ӦΪ��
2ZnS+3O
22ZnO+2SO
2�� �ķ�¯�У�2C+O
22CO ZnO+CO
Zn+CO
2�ӻ���п�����IJ����к����ֽ������ʼ�In
2O
3������������ȡ����ij�о������Դ��о��������£�ʵ�����漰����ȣ�ÿ����Һ�к�����������������Ľ�������ͼ1��������Һ������������������ͼ2
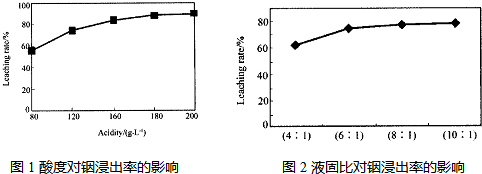
�ٵ����Ϊ196ʱ�������ʵ���Ũ��Ϊ
2mol?L-1
2mol?L-1
��
�ڴӽ�Լԭ�Ϻͽ����ʿ��ǣ����˵���Ⱥ�Һ�̱ȷֱ�Ϊ��
180
180
��
6��1
6��1
��
��2��ʪ����п����Ҫ��������Ϊ��

�ٴӱ��������ͳ������ԭ�ϽǶȣ���δ�������������
���̵����������������������
���̵����������������������
��
�ڳ�ȥ�����Һ�е���������H
2O
2�������ٵ���pHʹ֮�γ�Fe��OH��
3�������H
2O
2����Fe
2+�����ӷ���ʽ
2Fe2++H2O2+2H+=2Fe3++2H2O
2Fe2++H2O2+2H+=2Fe3++2H2O
��
�������Һ������Cd
2+��Ϊ�˷�ֹ����Ⱦ�������ӣ������������ʵIJ��죬��������������Һ���룬��֪Zn��OH��
2����������һ��Ҳ�������ԣ���д����������ӷ���ʽ
Cd2++2OH-=Cd��OH��2����Zn2++4OH-=ZnO22-+2H2O[��Zn2++4OH-=Zn��OH��42-]
Cd2++2OH-=Cd��OH��2����Zn2++4OH-=ZnO22-+2H2O[��Zn2++4OH-=Zn��OH��42-]
��






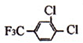 +
+
 +HCl
+HCl +
+
 +HCl
+HCl A�������۷�Ӧ
A�������۷�Ӧ +nH2O
+nH2O +nH2O
+nH2O �ĺ�����������F3Cһ���ͱ�����ͬ���칹�干��
�ĺ�����������F3Cһ���ͱ�����ͬ���칹�干��
