
��15�֣�
��֪��
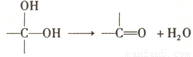
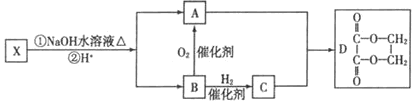
��ͼ��X�Ƿ���ʽΪC4H2Br2O4����Ԫ��������˴Ź������ױ����������ֻ��һ������Hԭ�ӵ����շ塣
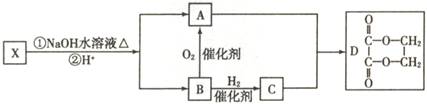
��B�����������ŵ����� ��
��X�Ľṹ��ʽ �������Է��� ������ĸ����
a��ȡ����Ӧ b����ȥ��Ӧ c���ӳɷ�Ӧ
��д��A��C��1:1��Ӧ���ɾۺ���Ļ�ѧ����ʽ ��
��D�ж���ͬ���칹�壬д��ͬʱ������������������һ��ͬ���칹��Ľṹ��ʽ ��
a������NaHCO3��Һ��Ӧ�ų����� b���ܷ���ˮ�ⷴӦ
c�������ں���һ����Ԫ��
��)����ȲΪ��Ҫԭ�Ϻϳ�HOCH2CHO���ϳ���Ҫ������д����������Ӧ�Ļ�ѧ����ʽ��������Ӧ������
��CH��CH + HBr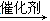 CH2��CHBr ��
CH2��CHBr ��
�� ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011���Ϻ��з���������4�µ��в��ԣ���ģ����ѧ�Ծ� ���ͣ������
���л�ѧѧϰ�Σ����Ĵ�������ӦҲ����ij����M�����ԭ��������100������������������MԪ�ص�������һ������������NH3��±���ӵȰ�ij�̶��������ν�ϳ��ȶ�����������ӣ�������ԭ���ţ���
��1��150�桢��ѹ�£�6.08g����������0.6mol HCl�����ַ������ֽⷴӦ����������ʣ�࣬�����Ϊ0.48mol���ý���M�����ԭ������Ϊ___________��
��������Ӧ���ù�������ˮ���50.0mL��Һ������Һ�����ʵ����ʵ���Ũ��Ϊ
_______mol��L-1��
��2��ȡ��1����������Һ12.5mL��ϡ����25mL������ͨ��2688mL����������£�����һ�������·�Ӧǡ����ȫ���õ�����B��Ħ������Ϊ260.5g/mol����������1.5mol/L��AgNO3��Һ�ζ����ﵽ�յ�ʱ����ȥAgNO3��Һ40.0mL����BͶ������ռ���Һ�У�δ����NH3���ݳ�����B�Ļ�ѧʽ�ɱ�ʾΪ ��
��3����֪����ͼ�У�L��λ����ȫ��ͬ��������һ��������[M(NH3)6-xClx]n����1��x��5����xΪ���������ṹ������ͼ��
����������ӹ���2�ֲ�ͬ�ṹ���������ӵ�ʽ��Ϊ ��
��4��һ�������£�3.04g�ý���������ǡ�ñ�ij��������Ч���൱��0.03mol O2���������ټ���0.05mol KCl����һЩ����մ�����K��M��Ԫ��ǡ����ȫ�γɺ������Σ�ʽ����360���������ڲ��������Ӹ�����Ϊ1:2������ε�ʽ���Լ����ʵ����ֱ�Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ������
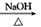 HOCH2CHO+3HBr
HOCH2CHO+3HBr �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����ͼ��B��GΪ������������A��JΪ�ǽ������嵥�ʣ�����Ϊ�������ʻ���ˮ��Һ������EΪ��ɫ������B��A������ȼ�ղ�����ɫ�̣�I����ɫ��ӦΪ��ɫ��FΪ��ɫ���壬���������Ϊ����ɫ��
�ش��������⣺
��1��д������D��ѧʽ ����ˮ��Һ�� �ԣ��������ӷ���ʽ˵��ԭ�� ��
��K�����������Ļ�ѧ���� �����е���ʽΪ ��
��2��д��B��C��Ӧ�����ӷ���ʽ ��
��3��1molG��������ˮ��Ӧ�����ɻ�ԭ���������Ϊ g��
��4��J����һ�ֳ�������ɫ���嵥����K��Һ�пɹ���һ��ȼ�ϵ�أ�д���õ�ظ����ĵ缫��Ӧʽ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
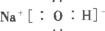
�ش��������⣺
(1)д�����ٻ�ѧʽD___________����K���ʵĵ���ʽ___________��
(2)д��B��C��Ӧ�����ӷ���ʽ______________________��
(3)1 molG��������ˮ��Ӧ�����ɻ�ԭ���������Ϊ___________g��
(4)��ʯīΪ�缫���L��Һ�Ļ�ѧ����ʽΪ___________������·��ת��4mol����ʱ���ɴ��������ռ�������___________L(��״��)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com