
 �������£�
�������£� �ϸ�ƿ��ʢ�ŵ��Լ�Ϊ���û�ѧʽ�ش𣩣���ÿ��1�֣�
�ϸ�ƿ��ʢ�ŵ��Լ�Ϊ���û�ѧʽ�ش𣩣���ÿ��1�֣� ��D ��
��D ��
 ��
�� ��
��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢ� | B���ܢ٢ڢ� | C���٢ۢڢ� | D���ܢڢ٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
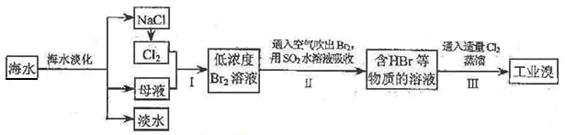
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
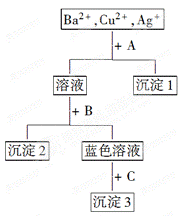

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
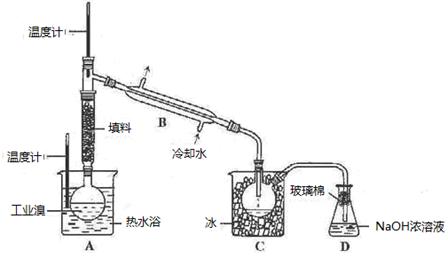
| ���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | �ܽ��� |
| �Ҷ��� C2H6O2 | -11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
| ������C3H8O3 | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO2����������SO2����ͨ�����Ը��������Һϴ�����ٸ��� |
B�� NaCl��Һ����������Na2SO4�����ӹ�����BaCl2��Һ���ٹ��� NaCl��Һ����������Na2SO4�����ӹ�����BaCl2��Һ���ٹ��� |
| C��NO����������NO2������ˮϴ�Ӻ��ٸ��� |
| D����ȥNa2CO3������������NaHCO3�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �� �� ��
�� �� �� aHSO4 ��Ca��OH�� 2 Na2CO3 BaCl2
aHSO4 ��Ca��OH�� 2 Na2CO3 BaCl2| A���٢� | B���ڢ� | C���٢ۢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
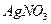 ��
��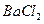 ��
��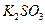 ��
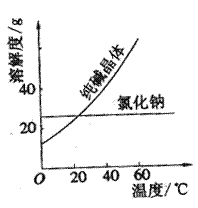
��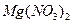 ������Һ������������֮������Ӧ�����Լ�����
������Һ������������֮������Ӧ�����Լ�����| A�����ᡢ���� | B�����ᡢ����������Һ | C����ˮ������ | D����ˮ������������Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com