
���� ��1������n=C•V��m=n•M�����㣻
��2������������Һ��ʵ���������ѡ�������������
��3�������������������ʵ�������Һ�����Ӱ�죬����c=$\frac{n}{V}$�ж϶�������ҺŨ�ȵ�Ӱ�죮
��� �⣺��1������500ml 0.1mol•L-1��Na2CO3��Һ��Ҫ��Na2CO3�����ʵ���n=C•V=0.5L��0.1mol•L-1=0.05mol������m=n•M=0.05mol��286g/mol=14.3g��
�ʴ�Ϊ��14.3��
��2�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܡ�ҩ�ף�
�ʴ�Ϊ��500mL����ƿ������������ͷ�ιܣ�
��3���ټ�ˮ�����˿̶��ߣ���Һ�����ƫ��������Һ��Ũ��ƫ�ͣ���Һ�Ǿ��ȵģ�ȡ��ˮʹҺ��ǡ�õ��̶��ߣ�ʣ����Һ��Ũ����ԭ��Һ��Ũ����ȣ���ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�����ǽ�ϴ��Һ��������ƿ�ᣬ��������ƿ�����ʵ����ʵ�����С��������Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
�۶�����Ҫ��ˮ������ƿ�ڱڸ���ˮ���δ���ﴦ������������Һ��Ũ����Ӱ�죬
�ʴ�Ϊ����Ӱ�죻
����Һ�������ˮ���ݣ�����ƿû�и����������Һ��Ũ����Ӱ�죬
�ʴ�Ϊ����Ӱ�죮
���� ���⿼��һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ�����c=$\frac{n}{V}$�ж�������Һ����ԭ������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �����Լ�A | Һ���Լ�B | |
| �� | ||
| �� | ||
| �� | ||
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ȼ����Ϊ285.8kJ•mol-1��������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g���T2H2O��l����H=-285.8 kJ•mol-1 | |
| B�� | ��֪�к���Ϊ57.3 kJ•mol-1������1L1mol•L-1�����뺬1molNaOH��Һ��ϣ��ų�������ҪС��57.3kJ | |
| C�� | Ba��OH��2•8H2O��s��+2NH4Cl��s���TBaCl2��s��+2NH3��g��+10H2O��l����H��0 | |
| D�� | ������������������Ʒֱ���ȫȼ�գ����߷ų��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
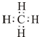 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
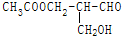 �й�ѧ���ԣ��������з�Ӧ�����ɵ��л�����ѧ���Ե��ǣ�������
�й�ѧ���ԣ��������з�Ӧ�����ɵ��л�����ѧ���Ե��ǣ�������| A�� | ����ᷢ��������Ӧ | B�� | ��NaOHˮ��Һ���� | ||
| C�� | ��������Һ���� | D�� | ����Br2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
 ��
��
| ��� | ͬλ�� | ͬ�������� | ͬ���칹�� |
| ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ��������������� | B�� | ֻ�������Ҵ������� | ||
| C�� | ֻ�������Ҵ����������������� | D�� | ֻ���������ᡢ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������������ƵĴ���Һ���ȣ�CH3CH2Br+NaOH$��_{��}^{��}$CH2=CH2��+NaBr+H2O | |
| B�� | ��������Һ������ȩ�еĹ����ţ�CH3CHO+2[Ag��NH3��2]++2OH-$\stackrel{ˮԡ����}{��}$CH3COO-+NH4++3NH3+2Ag��+H2O | |
| C�� | ������Һ��ͨ��������CO2��CO2+H2O+2C6H5O-��2C6H5OH+CO32- | |
| D�� | �ʰ���������������Һ��Ӧ��H2N-CH2COOH+OH-��H2N-CH2COO-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2 | B�� | SO2 | C�� | SO3 | D�� | NO2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com