
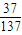 =117g��n��NaCl��=
=117g��n��NaCl��=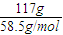 =2mol�����ݷ�Ӧ�ķ���ʽ��������NaHCO3������������ܽ�ȼ������������������
=2mol�����ݷ�Ӧ�ķ���ʽ��������NaHCO3������������ܽ�ȼ������������������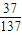 =117g��n��NaCl��=
=117g��n��NaCl��=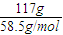 =2mol��
=2mol��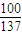 -2×18g=280g��
-2×18g=280g��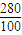 ×14��39.0g��
×14��39.0g��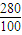 =22.8g��
=22.8g��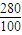 =92.4g��
=92.4g��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
"NaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl"��������"�����Ƽ"����Ҫ��Ӧ��������4λͬѧ�Ը÷�Ӧ�漰���й�֪ʶ�����IJ��ּ��⡣���в���ȷ���ǣ� ��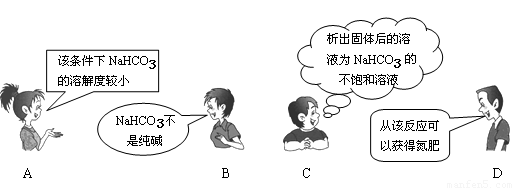
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ�������п��Ի�ѧ�Ծ� ���ͣ�ѡ����
��NaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl����������"�����Ƽ"����Ҫ��Ӧ��������4λͬѧ�Ը÷�Ӧ�漰���й�֪ʶ�����IJ��ּ��⡣���в���ȷ����
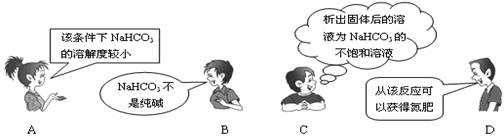
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl����������"�����Ƽ"����Ҫ��Ӧ��������4λͬѧ�Ը÷�Ӧ�漰���й�֪ʶ�����IJ��ּ��⡣���в���ȷ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com