��2010?�Ͼ���ģ�������������ƶ���������Ⱦ����Ҫ�����ֶΣ�
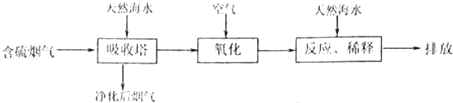
��1�����ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��
ij�о�С��Ϊ̽����ߺ���������SO
2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ1��ʾ��
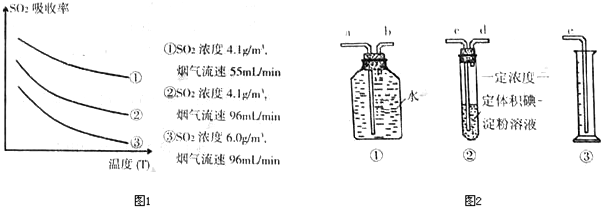
�ٸ���ͼʾʵ������Ϊ�����һ��Ũ�Ⱥ���������SO
2������Ч�ʣ����д�ʩ��ȷ����
ABD
ABD
��
A������ͨ�뺬���������¶ȣ�B����Сͨ�뺬������������
C��������Ȼ��ˮ�Ľ�������D������Ȼ��ˮ�м�����ʯ��
����Ȼ��ˮ�����˺��������������H
2SO
3��ʹ�ÿ����е���������������д���÷�Ӧ�����ӷ���ʽ
2H2SO3+O2=2H2SO4
2H2SO3+O2=2H2SO4
��
�۸�С�����ͼ2װ����ʵ���Ҳⶨ������SO
2���������������ʵ���ڱ�״���½��У�������װ����װ���ӵ�˳���ǣ�ԭ������
cdbae
cdbae
����a��b��c��d��e���������Լ��У�Ũ�ȡ����һ�������������������Թ��еĵ�-������Һ����
AC
AC
�����ţ���
A������KMnO
4��Һ��B��NaOH��Һ��C����ˮ��D����ˮ
��2��ʯ��ʯ-ʯ��ʪ�����������ռ����Ĺ���ԭ���������еĶ��������뽬Һ�е�̼����Լ�����Ŀ�����Ӧ����ʯ�ࣨCaSO
4?2H
2O����д���÷�Ӧ�Ļ�ѧ����ʽ��
2CaCO3+2SO2+O2+4H2O�T2��CaSO4?2H2O��+2CO2��
2CaCO3+2SO2+O2+4H2O�T2��CaSO4?2H2O��+2CO2��
��ij�糧��ú300�֣�ú�к�����������Ϊ2.5%������ȼ��ʱú�е���ȫ��ת���ɶ���������ʯ��ʪ��������������96%����ת��Ϊʯ�࣬�������ʯ��
38.7
38.7
�֣�





 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�